Chapter 25 - Aromatic Compounds
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
benzene
naturally occurring aromatic hydrocarbon
molecular = C6H6
empirical = CH
bond angle = 120
issues with Kekule’s model of benzene/why the delocalised model is more accurate
-benzene is less reactive than alkenes/does not readily react to addition
-enthalpy change of hydrogenation of benzene is less exothermic/more stable than expected
-all the carbon bond lengths are the same
-benzene is less reactive than alkenes/does not readily react to addition
-in benzene pi electrons are delocalised + in alkenes pi electrons are localised
-benzene has a lower electron density than alkenes so induces a weaker dipole in bromine so does not decolourise bromine water
-enthalpy change of hydrogenation of benzene is less exothermic/more stable than expected
-the enthalpy change of hydrogenation of cyclohexene to cyclohexane is -120kJmol-1
-so the theoretical enthalpy change of hydrogenation of benzene would be -120 × 3 due to 3 double bonds = -360kJmol-1
-however the actual enthalpy change was -208kJmol-1 which is 152kJmol-1 more energetically stable than expected
-all the carbon bond lengths are the same
-benzene should contain 2 different bond lengths as single bond length is 0.153nm and double bond length is 0.134nm
-but X-ray crystallography/diffraction revealed that the 6 carbon-carbon bonds were all of length 0.140nm
differences between Kekule’s model + delocalised model
-they both have p-orbitals overlapping to form pi bonds
-but in delocalised model the pi bonds are delocalised + in Kekule’s model the pi bonds are localised
Kekule’s model of benzene (DRAW STRUCTURE + BONDS)
.
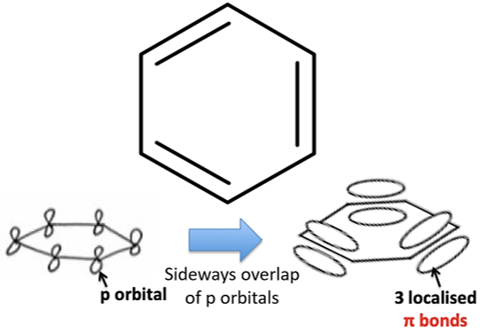
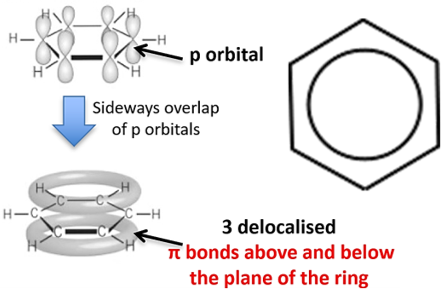
delocalised model of benzene (DRAW STRUCTURE + BONDS)
the 6 pi electrons in benzene are free to move between all 6 carbon atoms in the ring and are not localised within three distinct double bonds
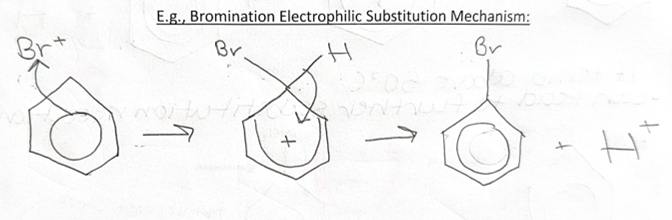
electrophilic substitution of benzene
an electrophile has substituted hydrogen from the benzene ring
-benzene has a high electron density above and below ring due to delocalised electrons
NITRATION reagents + reaction conditions
-concentrated sulfuric acid + concentrated nitric acid
-reflux at 50C
electrophilic substitution -NITRATION
stage 1 = creating a nitronium ion NO2+
1) H2SO4 + HNO3 ⇌ [H2NO3]+ + HSO4-
2) [H2NO3]+ ⇌ NO2+ + H2O
overall = H2SO4 + HNO3 → NO2+ + HSO4- + H2O
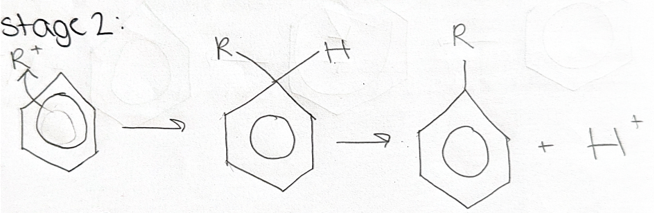
stage 2 = draw
stage 3 = acid catalyst regenerated
H+ + HSO4- → H2SO4
![<p><strong><u>stage 1</u></strong> = creating a nitronium ion <strong>NO2+</strong></p><p>1) <span style="color: blue">H2SO4 </span>+ HNO3 ⇌ [H2NO3]+ + HSO4-</p><p>2) [H2NO3]+ ⇌ <strong>NO2+</strong> + H2O</p><p>overall = <strong>H2SO4 + HNO3 → NO2+ + HSO4- + H2O</strong></p><p><strong><u>stage 2</u></strong> = draw</p><p><strong><u>stage 3</u></strong> = <span style="color: blue">acid catalyst</span> regenerated</p><p><strong>H+ + HSO4- → </strong><span style="color: blue"><strong>H2SO4</strong></span></p>](https://knowt-user-attachments.s3.amazonaws.com/b5425b07-38c1-4460-ad7c-bb9325859493.png)
HALOGENATION reagents + reaction conditions
-halogen carrier = AlCl3 or FeCl3, AlBr3 or FeBr3
-is needed as benzene has a lower electron density than an alkene so won’t be able to induce a dipole in bromine
-halogen
-room temp + pressure
electrophilic substitution -HALOGENATION
-bromination or chlorination
stage 1 = creating bromonium or chloronium ion Br+ or Cl+
Br2 + FeBr3 → Br+ + FeBr4-
stage 2 = draw
stage 3 = catalyst regenerated
FeBr4- + H+ → HBr + FeBr3
Friedel-Craft’s ACYLATION
stage 1 = creating acylium ion RCO+
RCOCl + AlCl3 ⇌ RCO+ + AlCl4-
stage 2 = draw
stage 3 = catalyst regenerated
H+ + AlCl4- → AlCl3 + HCl
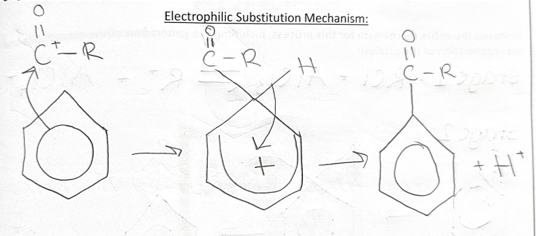
ACYLATION + ALKYLATION reagents + conditions
-acyl chloride for acylation OR haloalkane for alkylation
-halogen carrier
-reflux at 60C
-anhydrous conditions
Friedel-Craft’s ALKYLATION
stage 1 = RCl + AlCl3 ⇌ R+ + AlCl4-
stage 2 = draw
stage 3 = catalyst regenerated
H+ + AlCl4- → HCl + AlCl3
naming benzene compounds
1) stem + suffix = benzene
2) prefix - should have lowest number combination + in alphabetical order
3) for di- and tri- compounds, the first side chain is given the lowest number possible
side chain names
OH = hydroxy
NO2 = nitro
NH2 = amino
CN = cyano
benzene side chain = phenyl- - when H atom is removed from other functional group and replaced with benzene ring
phenol weak acid properties
1) partially soluble in water - OH group can form hydrogen bonds with water but aromatic group is non-polar so cannot
2) weak acid - orange - partially dissociates - more acidic than alcohols but less acidic than carboxylic acids
3) neutralisation reaction with NaOH + dissolves - can react strong bases such as NaOH to form a soluble salt and water
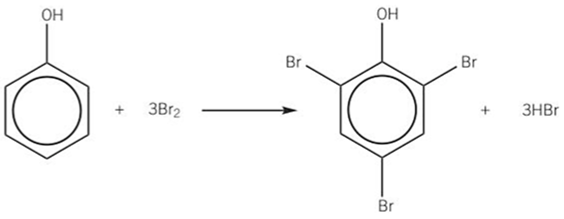
electrophilic substitution of phenol -DRAW TOO
-orange solution to a white solid precipitate in a colourless solution
-trisubstitution of bromine occurs and forms 2, 4, 6-tribromophenol
reaction conditions = room temp + pressure, halogen carrier NOT required
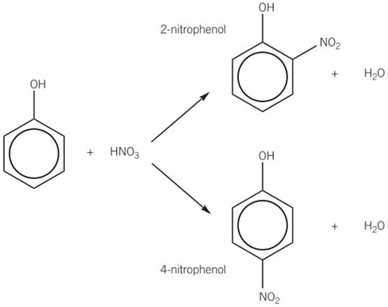
nitration of phenol -DRAW TOO
-reaction conditions = dilute nitric acid, no sulfuric acid, room temp + pressure
-trisubstitution does not occur = the nitro group (NO2) is a deactivating group
how is phenol more reactive than benzene?
-benzene + phenol contain delocalised pi electrons
-but the lone pair of electrons on oxygen from OH group on phenol is partially delocalised into the ring
-the OH group then activates the aromatic ring so phenol has a greater electron density
-so phenol is able to induce a dipole in bromine + is more susceptible to electrophilic attack while benzene cannot
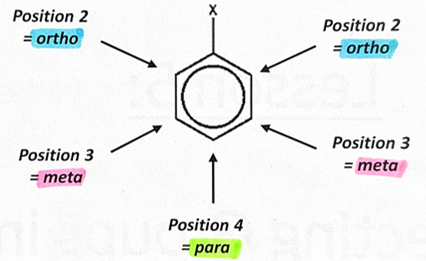
activating groups
-push electrons into the delocalised ring so increases electron density making molecule more reactive so reacts more readily with electrophiles
-they direct the group to the 2, 4 and 6 positions (2 and 6 are the same)
examples = -NH2, -OH
deactivating groups
-pulls electrons out of the delocalised ring and towards themselves so decreases electron density making molecule less reactive so reacts less readily with electrophiles
-they direct the group to the 3 and 5 positions (3 and 5 are the same)
example = -NO2