Chapter 31 - Air and Water Pollution
1/24
Earn XP
Description and Tags
Exam 3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
1) Ozone
2) Sulfur Dioxide
3) Particulate matter
4) Nitrogen Dioxide
5) Carbon monoxide
6) Lead
What are the six air pollutants ubiquitous to industrial communities and thought to carry the greatest risk to humans and environmental health?
Primary Standards
Provide public health protection, including protecting the health of “sensitive” populations such as asthmatics, children, and the elderly.
Secondary Standards
Provide public welfare protection, including protection against decreased visibility and damage to animals, crops, vegetation, and buildings.
Reducing-Type Air Pollution
Pollution that occured most often during the winter periods of oil and coal combustion and meteorological inversions.
ex) Sulfur dioxide
Oxidative-Type Air Pollution
Pollution that occurred during the summer time, when sunlight is most intense and can catalyze reactions among the constituents of auto exhaust.
ex) Ozone
Sulfur Dioxide (SO2)
A water-soluble irritant gas that is also a reducing pollutant. It is predominantly absorbed in the upper airways and stimulates bronchoconstriction and mucus secretion.
Sulfuric acid fume/sulfuted fly ash/protonating
Fill in the blank…
During oil and coal combustion or the smelting of iron ore, sulfur dioxide condenses with available metal ions and water vapor to form ( ) and ( ).
Sulfuric acid is capable of ( ) receptor ligands and other biomolecules, directly damaging membranes or initiating inflammation.
Particulate Matter
In the atmosphere, this pollutant can be solid, liquid, or a combination of both with a mixture of organic, inorganic, and biological compounds. Its compositional matrix can vary significantly depending on the emission source and secondary transformations, including gas to particle conversions.
Carbonaceous/sulfates/metals/silicates
Fill in the blank…
The major constituents of particulate matter include incompletely burned ( ) materials, acid ( ), various ( ), and ( ) associated with the solid nature of the fuel.
Lung/solubility/electron transfer
Fill in the blank…
Several metals and silicates that make up much of the inorganic phase of particulate matter can be cytotoxic to ( ) cells. ( ) plays a role in the toxicity of many inhaled metals by enhancing metal bioavailability. Some can promote ( ) to form reactive oxidants.
Ultrafine Carbonaceous Matter
These ultra fine particles in particulate matter typically result from high temperature oxidation or atmospheric transformation involving vapors and sunlight. Elemental carbon is diesel particular matter frequently combines into complex, long ultrafine chains and has the potential to act as a carrier of certain irritant gases.
Ozone
An oxidizing pollutant found at ground level and in the upper regions of the atmosphere. In these upper regions, UV light splits molecular oxygen into atomic O, which then combines with O2 to form this compound. It forms a thin strip in the stratosphere that serves as an effective permanent barrier by absorbing short wavelength-UV in the chemical process.
Nitrogen oxides/UV/toxic
Fill in the blank…
Ground level ozone is formed through a series of complex chemical reactions between ( ) and volatile organic compounds activated by the ( ) spectrum of sunlight. This ozone is ( ).
Less/greater
Fill in the blank…
The Ozone Monitoring Instrument (OMI) measures the ratio of formaldehyde to nitrogen dioxide. If this ratio is ( ) than 1, ozone will decrease when emissions of anthropogenic VOCs are reduced. If the ratio is ( ) than 2, ozone will decrease when nitrogen oxides are reduced.
Radicals/water
Fill in the blank…
Ozone is a toxicant of great concern; it is highly reactive and more toxic than nitrogen oxide, its generation is fueled by cyclic hydrocarbon ( ), and it reaches greater concentrations than the hydrocarbon radical intermediates.
Due to low ( ) solubility, a substantial portion of inhaled ozone penetrates deep into the lung. It can trigger chest pain, coughing, congestion, and worsening asthma/bronchitis. Repeated exposure can permanently scar lung tissue.
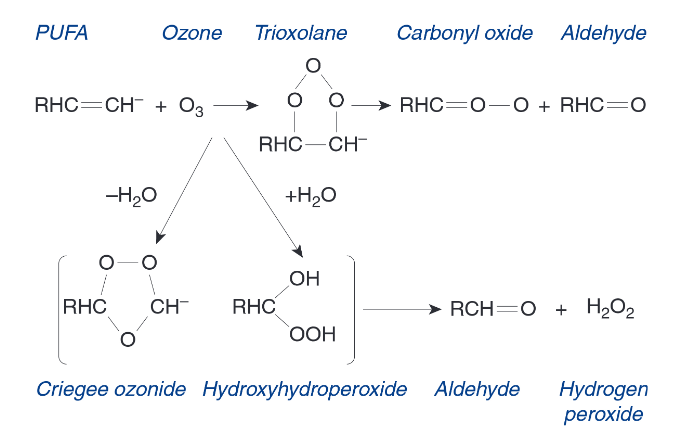
Polyunsaturated Fatty Acids (PUFA)
Fill in the blank…
The surface fluid lining the respiratory tract and cell membranes the underlie the lining fluid contain a significant quantity of ( ), either free or as part of the lipoprotein structures of the cell. These react with ozone to form toxic intermediates.
Nitrogen Dioxide
A pollutant that forms quickly from emissions of cars, trucks, and buses as well as from power plants and odd-road equipment. They are by-products of burning gasoline, in that the nitrogen molecules are broken down in the high temperatures of an engine and react with oxygen from thea ir to form the compound.
Nitric acid/acid rain
Fill in the blank…
Nitrogen oxides form ( ), a strong acid, when mixed with water and contribute to ( ).
Deep lung/less/indoor/neutrophilic
Fill in the blank…
Nitric oxide, like ozone, is a ( ) irritant that can produce pulmonary edema. It is a much ( ) potent irritant and oxidant that ozone, but it poses clear toxicological problems. It is also an important ( ) pollutant, especially for homes with unventilated gas stoves, kerosene heaters, or smokers.The pattern of injury is quite similar to that of ozone, but nitric oxide doesn’t pose as serious of a ( ) inflammation risk.
Carbon Monoxide
A chemical asphyxiant that exerts its toxic action through the formation of carboxyhemoglobin, which prevents oxygenation of the blood for systemic transport. The normal concentration of carboxyhemoglobin is very low, but this compound leads to a much higher equilibrium value.
Lead
A metal found naturally in the environment as well as in manufactured products.
Peroxyacetyl Nitrate (PAN)
Oxidant pollutant
A compound that is thought to be responsible for much of the eye-stinging activity of smog. It is one of the more common air pollutants formed by the action of sunlight on VOCs and nitrogen oxides. It is more water-soluble and reactive than ozone, so it rapidly decomposes in mucous membranes before it can penetrate deeply into the respiratory tract.
It has similar toxicity to nitrogen dioxide, higher than sulfur dioxide and less than ozone.
Aldehydes
Oxidant pollutants
These are carbonyl compounds, mostly short-chained, that are common photo-oxidation products of unsaturated hydrocarbons. Two major forms found in tobacco smoke are of interest: formaldehyde and acrolein.
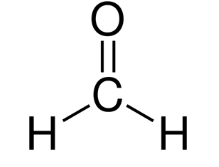
Formaldehyde
A primary sensory irritant that is very soluble in water and is absorbed in the mucous membranes of the nose, upper respiratory tract, and eyes. Its dose-response curve is steep and is ubiquitously present in indoor atmospheres due to plywood and furniture.
It is both a potential allergen and carcinogen.
Acrolein
An unsaturated aldehyde that is more reactive than formaldehyde. It penetrates deeper into airways and it may not have the same degree of sensory irritancy, but it may cause more damage. Slightly stiffened airways may result from the protein cross-linking action of this compound.