Equilibrium constant Kp
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
Define total pressure
The total pressure in a reaction is the sum of all the pressures of the individual gases (partial pressure)
What is a mole fraction?
The partial pressure of gas is calculated using mole fractions
A mole fraction is the proportion of a gas in a gas mixture
How do you workout the mole fraction of a gas
number of moles of gas/total number of moles of gas in the mixture
How do you workout partial pressure
Mole fraction of a gas x total pressure in mixture
Kp expression
(partial pressure of products)/(partial pressure of reactants)
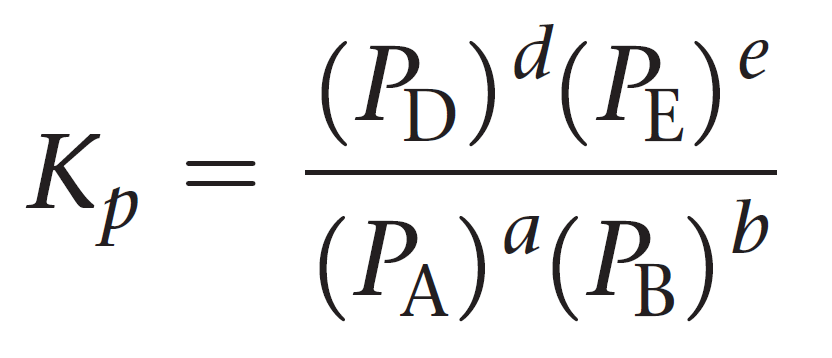
Units for Kp
Depends
Write the units top and bottom and cancel. Invert the powers if there is more units under the line.
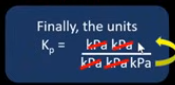
What affects the value of Kp
Temperature
How does temperature cause Kp to change
If temperature change causes equilibrium to shift right. Kp will increase
If temperature change causes equilibrium to shift left Kp will decrease