6.3.2 Spectroscopy
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What are the two types of NMR spectroscopy?
Carbon-13
1H (proton)
Give the 3 requirements for NMR spectroscopy
Strong magnetic field provided by electromagnet
Sample dissolved in suitable solvent and a bit of TMS
Low frequency radiowaves
What is nuclear spin? What is the relevance of this to NMR?
Just like electrons, the nucleus will have a nuclear spin which is especially significant with an odd number of nucleons (neutrons and electrons)
Therefore 1H and 13C are used as they contain odd number of nucleons
What is resonance? What is the relevance of this to NMR?
Like electrons, nuclei have 2 different flip states with different energies
When the nucleus absorbs the right combo of strong magnetic field and radiowaves, the nucleus can absorb enough energy to flip between flip states (resonance)
NMR measures the frequency of radiowaves absorbed for resonance to occur
What does the frequency required for the NMR spectrometer depend on?
The electromagnetic field strength
The small quantity of energy can only be detected in strong and uniform magnetic fields
What is chemical shift?
The scale used to compare frequency of NMR absorption with the frequency of the reference peak of TMS at delta = 0ppm
I.e. place in NMR spectrum at which a nucleus absorbs energy for resonance to occur
What is NMR calibrated by and what are the units?
By the delta scale
Units are ppm
What is TMS and what is its use in NMR?
Tetramethylsilane, (CH3)4Si - the standard reference chemical against which all chemical shifts are measured
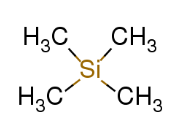
What is the chemical shift of TMS and why?
0 ppm as it contains 12 equivalent protons (H+), giving it a single shapr NMR signal at 0
What are the properties of TMS?
Volatile so can evaporate from sample after running NMR
Chemically unreactive
Give the process of running the NMR:
Sample is dissolved in solvent and a bit of TMS, then placed in narrow NMR sample tube
Placed in NMR spectrometer and spun to even out imperfections in magnetic field in the sample
Spectrometer zeroed against TMS standard and sample is given pulses of radiation
Absorption of energy from resonance are detected and displayed
Solvent and TMS is evaporated from sample
What is the issue with using normal solvents for NMR?
They contain carbon and hydrogen atoms which would produce a signal
Which 2 solvents are used for NMR?
Deuterated compounds:
Deuterium (2H) - stable isotope of hydrogen with an even number of nucleons
1 proton and 1 neutron
Deuterated trichloromethane (CDCl3)
Will produce a peak in the carbon-13 NMR but the peak is identified and filtered out by the computer