L13: Anaerobic respiration
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What determines how much energy a bacterium can gain from its electron transport chain (ETC)?
The difference in reduction potentials between the electron donor and terminal electron acceptor. A larger potential difference produces more energy.
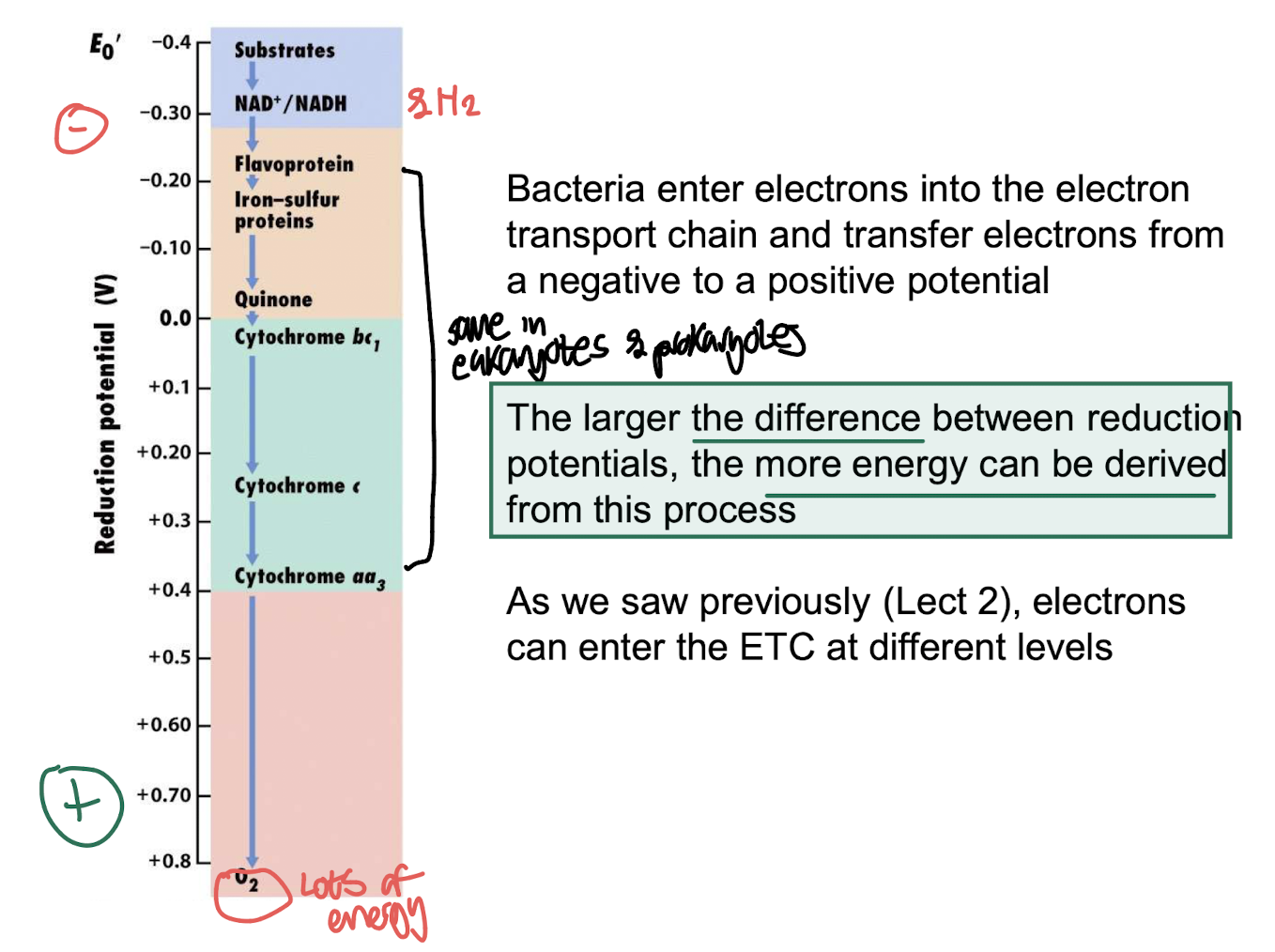
Why do different electron entry points into the ETC result in different energy yields?
Because electrons entering at different levels cause different numbers of protons to be pumped, changing the proton motive force and the resulting ATP production.
Which terminal electron acceptor yields the highest energy in respiration?
O₂, because the O₂/H₂O couple is the most electropositive, generating the strongest proton motive force when electrons flow from NADH to O₂.
The O₂/H₂O redox pair is the most electropositive, allowing bacteria to generate the largest ________.
proton motive force.
What is the difference between obligate aerobes, obligate anaerobes, and facultative aerobes?
Obligate aerobes: use only O₂.
Obligate anaerobes: cannot use O₂.
Facultative aerobes: can use O₂ and alternative electron acceptors
What electron acceptor does E. coli prefer when multiple are available? Why?
O₂, because it yields the most energy—similar to catabolite repression in carbohydrate metabolism.
What is the difference between assimilative and dissimilative reductions?
Assimilative: reduces compounds (e.g., NO₃⁻, SO₄²⁻) for biosynthesis—product incorporated into cell material.
Dissimilative: reduces compounds for energy generation—product excreted.
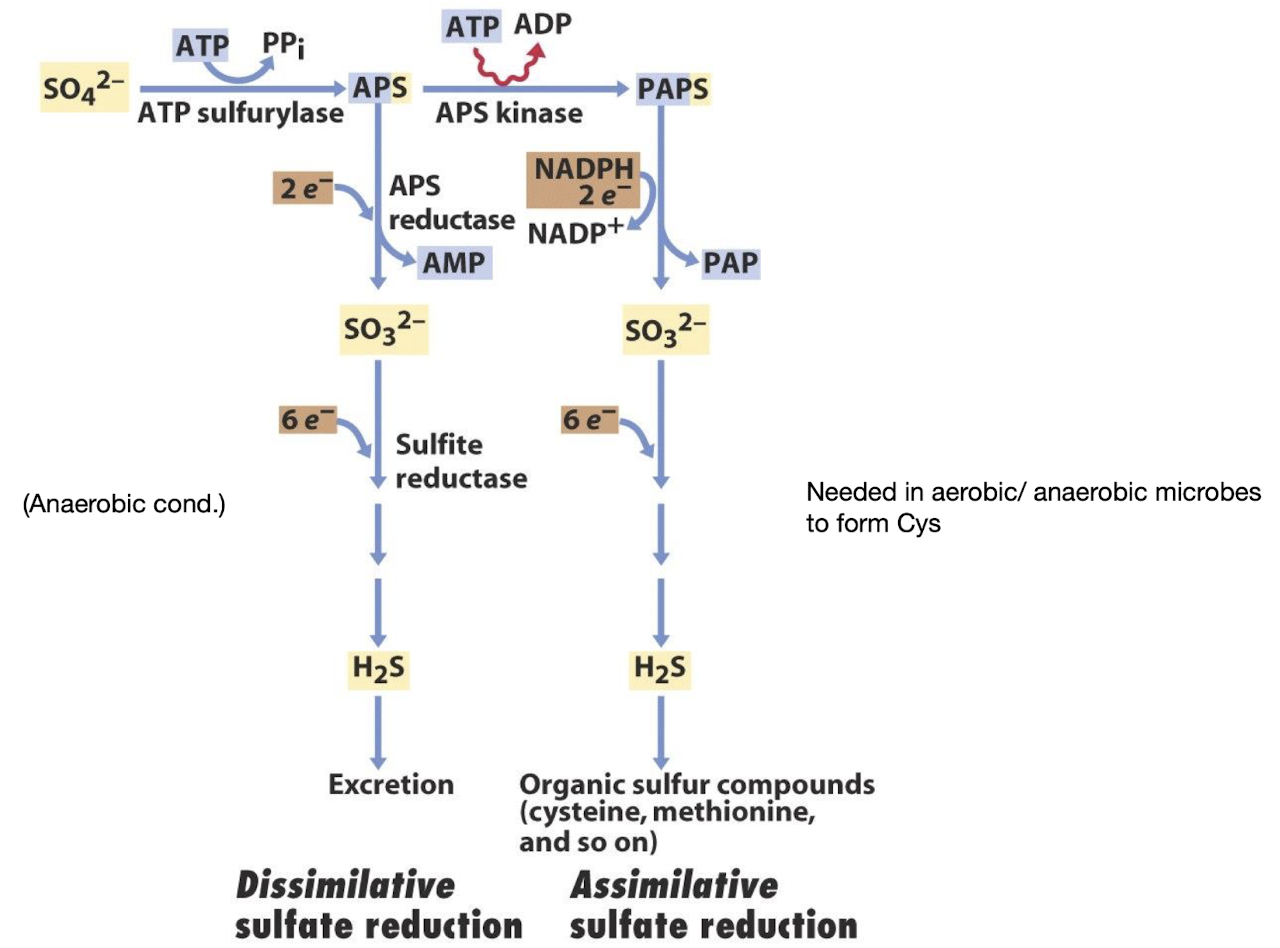
Give an example of assimilative and dissimilative sulfur metabolism
Assimilative: reduction of SO₄²⁻ → into R-SH e.g. in cysteine or methionine inside the cell.
Dissimilative: SO₄²⁻ → H₂S (excreted)

Assimilative reductions produce metabolites used in ________, while dissimilative reductions release products for ________.
biosynthesis; energy generation.
What is nitrification?
The aerobic oxidation of NH₄⁺ → NO₂⁻ → NO₃⁻ performed by chemolithotrophs such as Nitrosomonas and Nitrobacter.
Nitrosomonas vs Nitrobacter
Nitrosomonas
Oxidizes ammonia (NH₃ → NO₂⁻)
Nitrobacter
Oxidizes nitrite (NO₂⁻ → NO₃⁻)
Why is nitrification a low-energy process?
The redox potential differences (NH₃/NO₂⁻/NO₃⁻) are small, yielding limited ATP.
What key enzymes oxidise ammonia to nitrite?
Ammonia monooxygenase (NH₃ + O₂ + 2H+ + 2 e-→ NH₂OH (formation of hydroxylamine) + H2O).
Hydroxylamine oxidoreductase (NH2OH + H2O NO2
- + 5H+ + 4e-)
Note that 2 e- are required in the 1st reaction
These are obtained from the electrons obtained in the second reaction
Only 2 electrons can be donated to the ETC
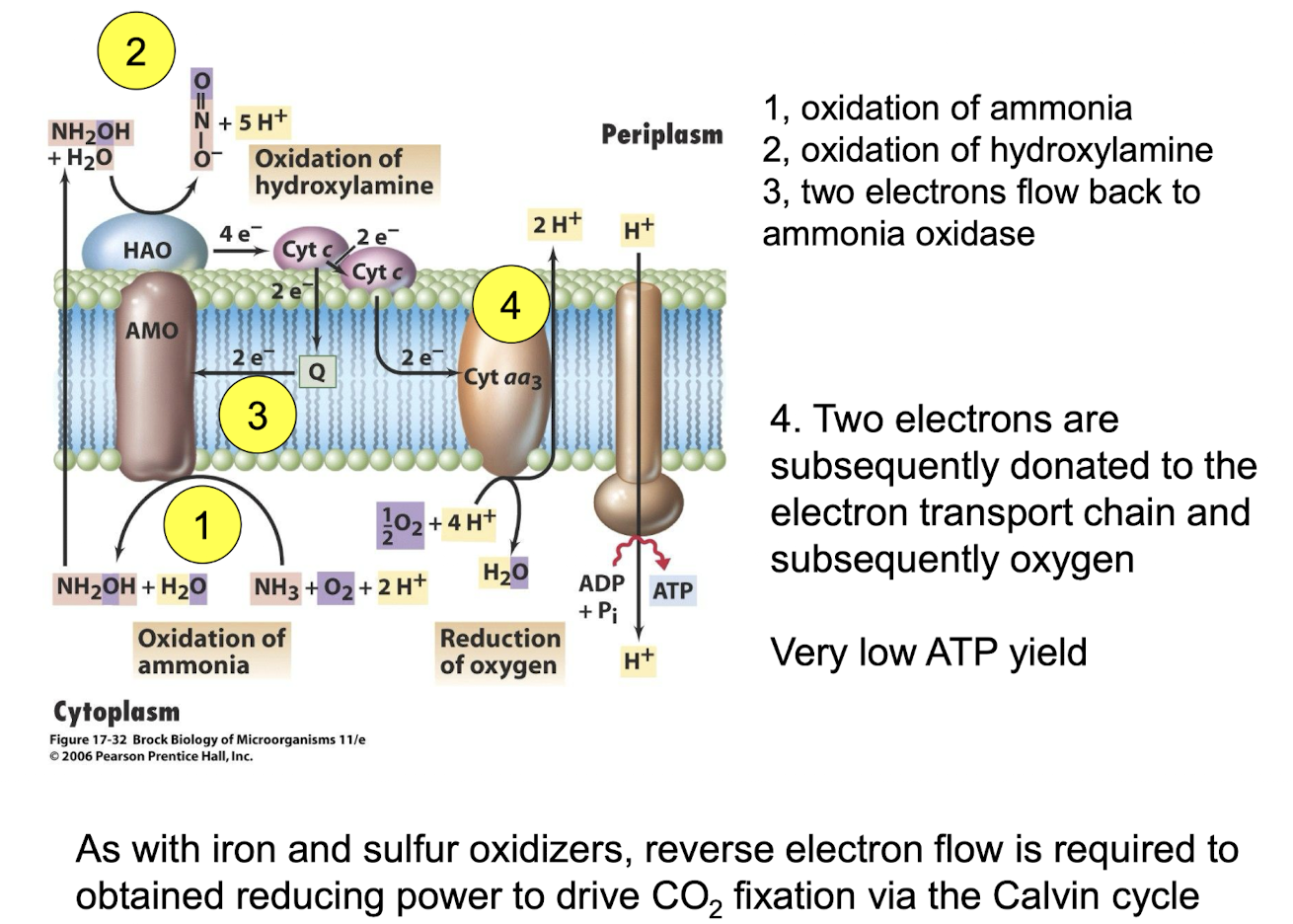
At what two stages is oxygen required for ammonia oxidation?
In the initial oxidation of ammonia
As a terminal electron acceptor
What is ANAMMOX and what bacteria are capable of it?
ANAMMOX (Anoxic Ammonia Oxidation) is a process where ammonia and nitrite are converted into nitrogen gas under anaerobic conditions.
bacteria capable of ANAMMOX include Planctomycetes
Has huge implications in wastewater treatment: ability to get rid of ammonia and nitrite without oxygen yielding only gaseous nitrogen (N2)
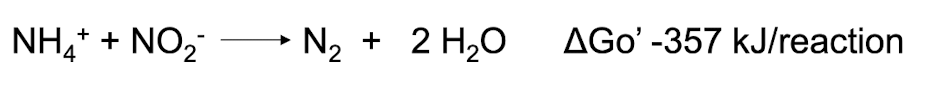
What is denitrification and why is it important?
The anaerobic reduction of NO₃⁻ → N₂.
Importance:
Removes nitrate from ecosystems (soil, water).
Used in wastewater treatment.
Prevents nitrate accumulation that can cause human health issues i.e. blue baby syndrome
What medical condition is linked to nitrate-to-nitrite conversion in infants?
Blue baby syndrome — nitrite converts hemoglobin (Fe²⁺) to methemoglobin (Fe³⁺), reducing O₂ transport and leading to oxygen deprivation in infants.
Why is nitrite dangerous besides methemoglobin formation?
NO₂⁻ is a potent carcinogen
What happens in the 5 stages of the sequencing batch reactor (SBR)?
Fill: aerobic mineralization begins (nitrification - conversion of NH4 to NO2/NO3).
React: continued aeration and nitrification.
Settle stage: sludge is allowed to settle clarify, no aeration; denitrification occurs (conversion of NO2/NO3 → N2)
Decant: treated supernatant removed.
Idle: sludge handling.
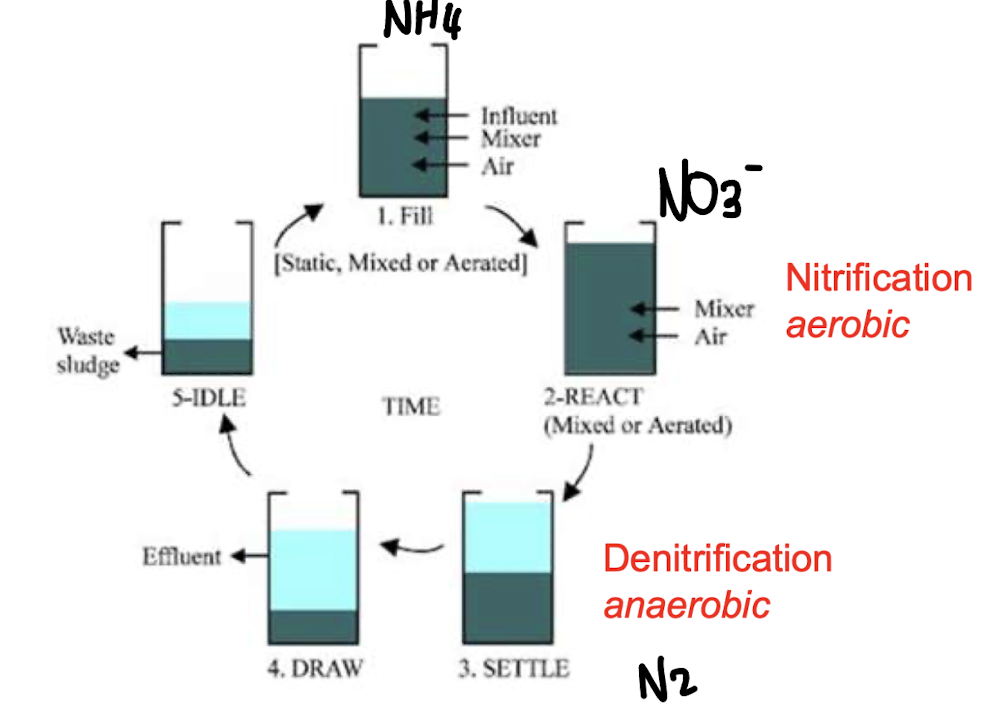
Nitrification occurs in ______ conditions, while denitrification occurs in ______ conditions
aerobic; anaerobic.
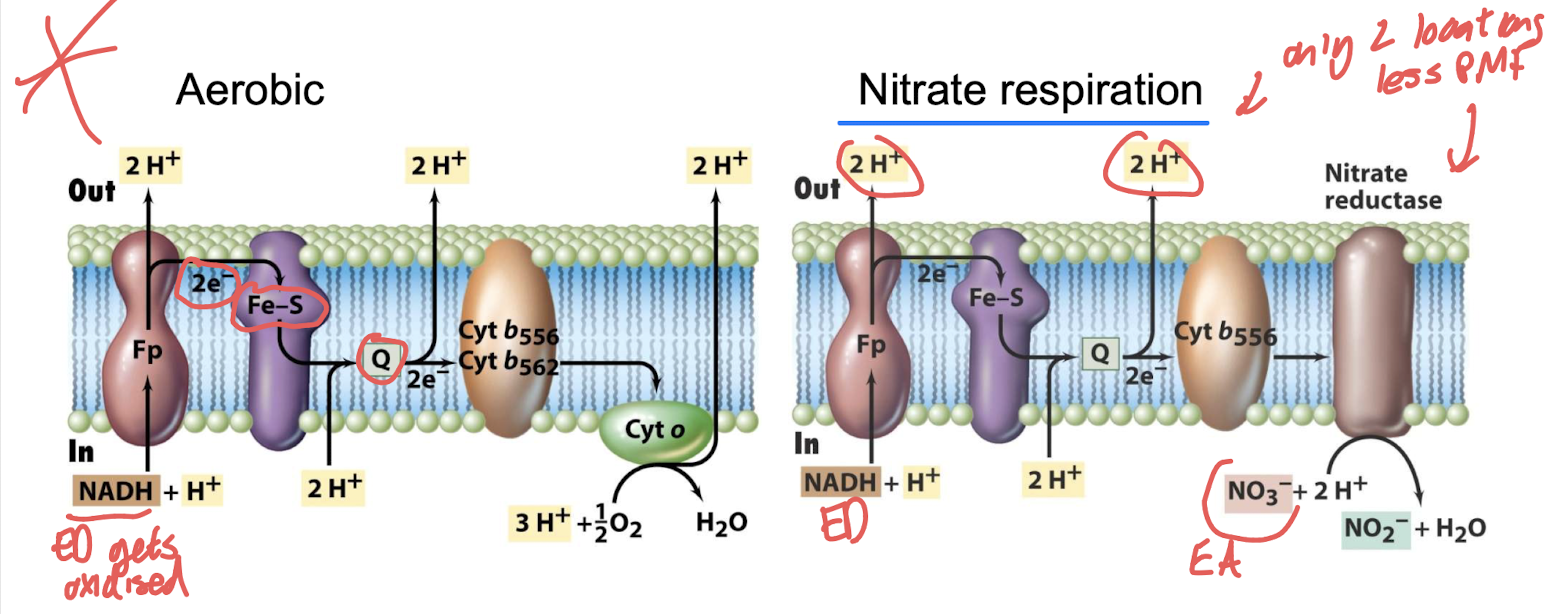
What enzyme catalyzes the final step in nitrate respiration?
Nitrate reductase — repressed by O₂ and induced by NO₃⁻.
Facultative aerobic bacteria use same electron transport chain.
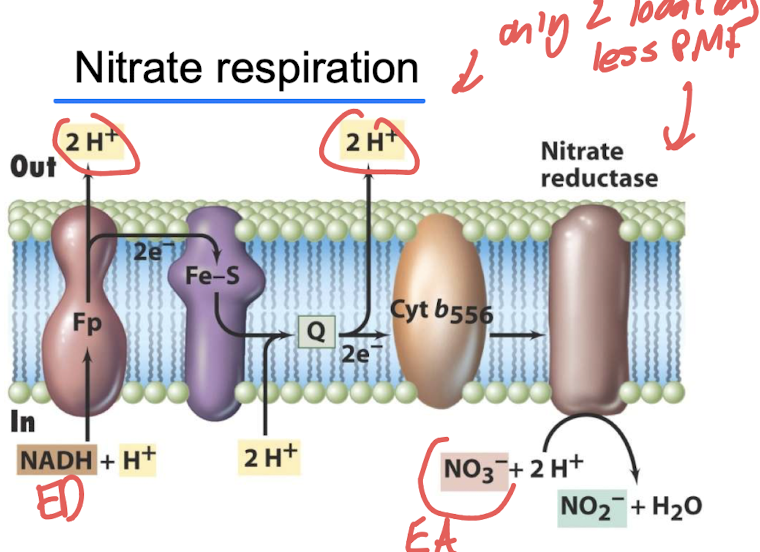
How does proton translocation differ between aerobic and nitrate respiration? .
Aerobic respiration: multiple proton-pumping steps.
Nitrate respiration: only two proton-translocating steps
Nitrate reductase synthesis is repressed by oxygen and induced by
nitrate
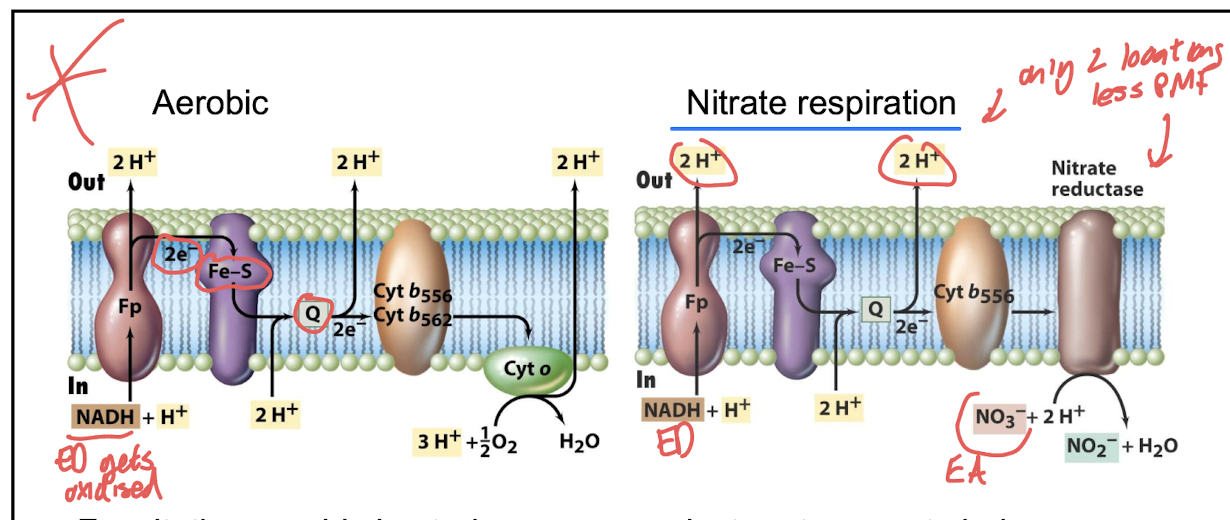
What is the advantage of an additional proton pump in further denitrification i.e. by Paracoccus denitrificans
The additional proton pump in Paracoccus denitrificans allows for greater ATP production during the denitrification process when NO3 is converted to N2, enhancing energy efficiency.
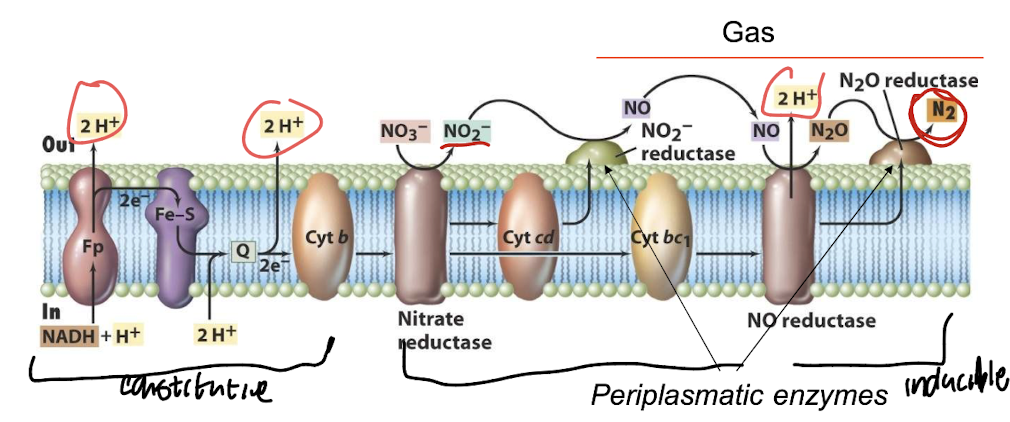
What harmful gas is produced during denitrification and why is it environmentally significant?
Gaseous NO is reduced to N₂O (nitrous oxide) — a potent greenhouse gas that also contributes to ozone depletion, when converted by sunlight
Why do sulfate-reducing bacteria have lower growth yields than nitrate or ferric iron reducers?
SO₄²⁻ is a much less favorable electron acceptor, producing less energy.
Where are sulfate-reducing bacteria commonly found?
Marine sediments where sulfate is abundant.
They produce FeS (black layer) and can lead to FeS₂ (pyrite) formation.
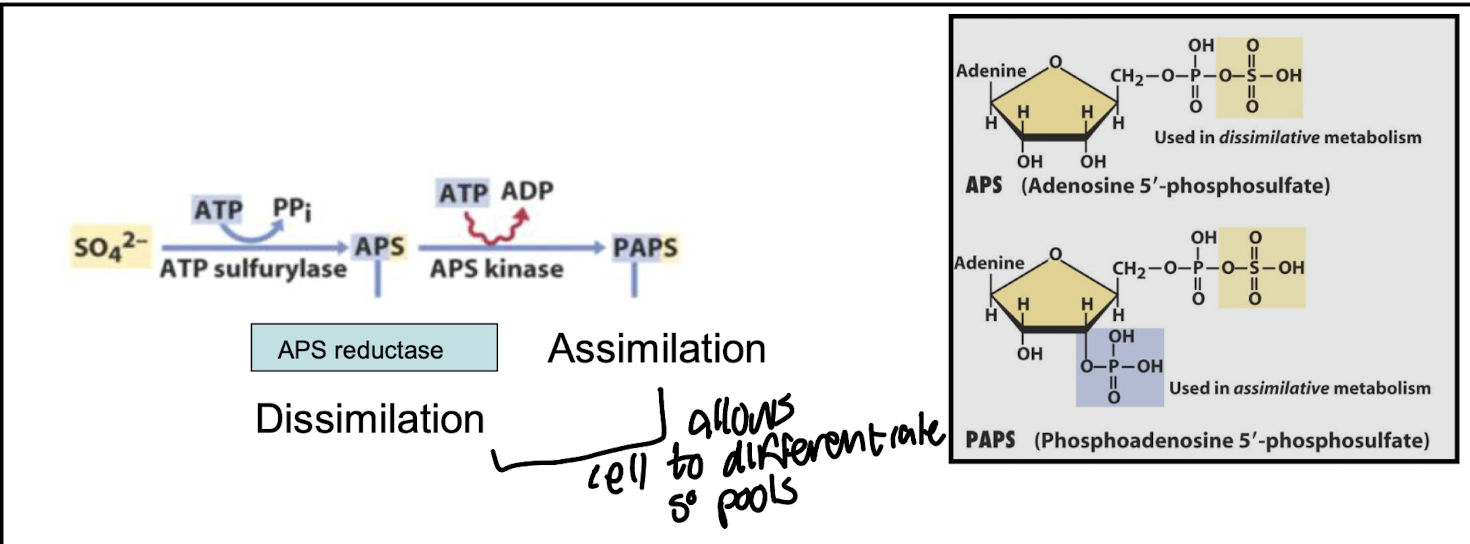
What enzyme activates sulfate before reduction?
ATP sulfurylase, forming APS (adenosine phosphosulfate).
Reaction: SO₄²⁻ + ATP → APS + PPi
What determines whether APS goes into assimilative or dissimilative pathways?
Regulation of APS kinase and APS reductase, allowing separate control of biosynthesis vs. energy generation.
The cellular demand for sulfate and the availability of electron donors influence its direction, determining the use of APS for either biosynthetic processes or energy production.
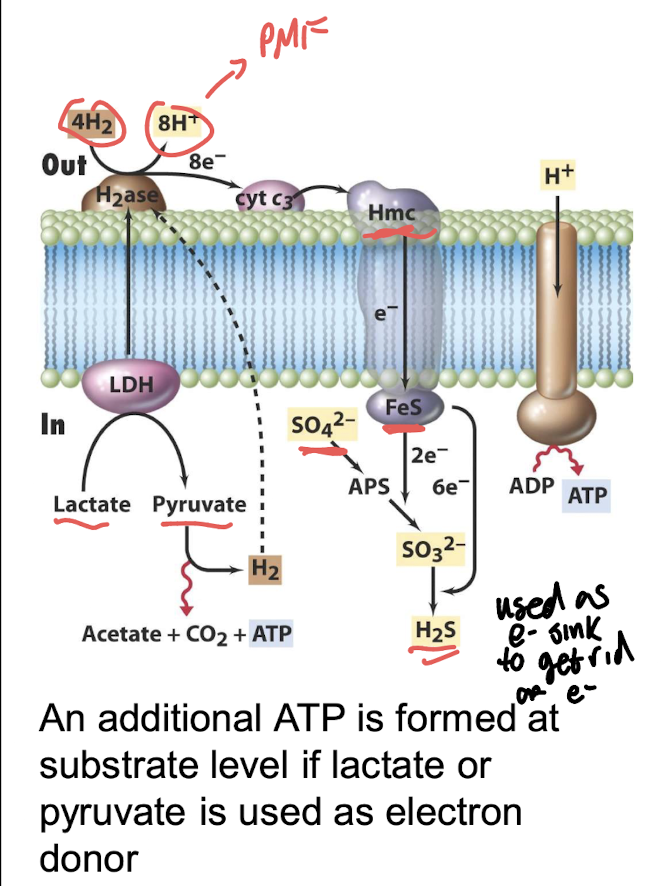
What electron donor is key in dissimilative sulfate reduction?
H₂ (via the Hmc transmembrane complex).
Overall reaction:
4H₂ + SO₄²⁻ + 2H⁺ → H₂S + 4H₂O
Sulfate must first be activated to ______ before reduction can occur.
APS (adenosine phosphosulfate).
What metabolic strategy parallels the control of assimilative vs. dissimilative sulfate reduction?
The glyoxylate bypass, regulated by controlling isocitrate lyase and isocitrate dehydrogenase.
Cells induce glyoxylate bypass during growth on fatty acids
What is the glyoxylate bypass and its metabolic purpose?
A CAC bypass that preserves carbon skeletons by producing malate from isocitrate via isocitrate lyase, replenishing CAC intermediates for biosynthesis.

How does the glyoxylate bypass replace finite CAC intermediates?
Isocitrate lyase cleaves isocitrate → succinate and glyoxylate
Glyoxylate condenses with acetyl CoA to form malate.
Contributes a new CAC intermediate molecule
Instead of being oxidised to 2 CO2 molecules, the 2-C glyoxylate fragment condenses with another acetyl CoA to form the 4-C compound malate.
Increases levels of all CAC intermediates, because malate can be oxidised to oxaloacetate that condenses with yet another acetyl CoA to form citrate, and so on.
Cells growing on acetate inhibit the enzyme ___________ to force carbon through the glyoxylate cycle
isocitrate dehydrogenase
This inhibition allows the diversion of carbon flow toward the production of glyoxylate and malate, facilitating the utilization of acetate.