Transport processes - diffusion, internal friction, heat conductivity.
1/41
Earn XP
Description and Tags
Haemodialysis, heat therapy and cryosurgery.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
What is diffusion?
Net movement of molecules or atoms from a region of high concentration (high chemical potential) to a region of low concentration (low chemical potential)
What type of process is diffusion?
Spontaneous process (no input energy is required) it can also be called as a passive process.
What is the quantitative mass transferred of a substance?
J = -D . dc/dx or M = -D . dc/dx . S.t
M - mass flowing per second through unit area
D - diffusion coefficient
dc/dx - concentration gradient
What is the gradient?
Vector quantity representing the rate of change over the distance.
Its value equals the difference in quantity’s values in two points from low to high.
What can diffusion be used for in medicine?
Haemodialysis
How does haemodialysis utilise diffusion?
Dialysis is the physical basis of haemodialysis, dialysis is the process of separating colloids from a solution of other dissolved substances through selective diffusion (waste removal) and convection (fluid removal) across a semi-permeable membrane
What can pass through the semi-permeable membrane?
Small molecules & ions - with small molecular mass
Impermeable for larger compounds
What does haemodialysis remove from blood?
Waste products, mainly nitrogen compounds (urea, creatinine, uric acid, guanine compounds, aromatic amines and medium sized molecules)
Process of haemodialysis
Toxin molecules diffuse through membrane separating two counter-fluxes — blood/dialysis fluid
Permanent flow of dialysis fluid provides a high conc gradient/difference
Basic steps to heamodialysis
Blood from patient is removed from the body through a large artery by a blood pump
Blood is sent to dialysis column
Direct blood purification is performed in the dialyser where the blood and dialysis solution are transfused
Molecules & toxins pass through membrane which is permeable for them and into the solution
Blood proteins & elements do not pass through membrane, remain in blood vol
Purified blood returns into patient’s body via a large vein
What tests are conducted & what is the result if the test is positive?
Tested for presence of haemoglobin
Tested for presence of gas bubbles
If test result is positive (haemoglobin present or gas bubble present) haemodialysis is stopped.
What is the risk associated with gas bubbles in haemodialysis?
Gas bubbles can lead to air embolism, which may cause stroke or cardiac arrest
Bubbles can obstruct blood flow in small vessels, resulting in tissue ischemia.
What does the presence of haemoglobin in dialysate indicate?
It may indicate haemolysis, which is the destruction of red blood cells.
Can occur due to factors like inappropriate dialyzer use, high blood flow rates, or incorrect anticoagulation.
Haemolysis can lead to complications such as anaemia, hypotension, and potential kidney damage.
What is internal friction?
A physical quantity equal to the product of velocity and mass of a moving object.
p=m⋅v
Momentum (p): A measure of the motion of an object, defined as the product of its mass and velocity. It is a vector quantity, meaning it has both magnitude and direction.
Mass (m): The amount of matter in an object, measured in ‘kg’.
Velocity (v): The speed of an object in a specific direction, measured in ‘m/s’.
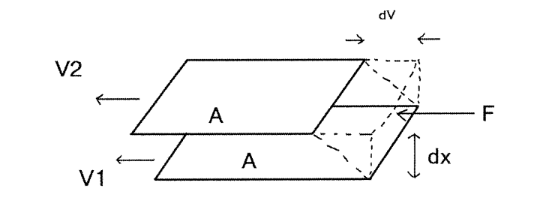
What is the equation for internal friction?
F=−η⋅dv/dx⋅S⋅t
Describes the force due to internal friction (viscous force) in a fluid
F: Force exerted by internal friction (viscous force).
η: Dynamic viscosity of the fluid (a measure of its resistance to flow).
dv/dx: Velocity gradient (rate of change of velocity across a distance perpendicular to the flow direction, also called shear rate).
S: Surface area over which the force acts.
t: Time duration over which the force is considered.
The equation essentially tells us that the internal friction in a fluid depends on how "thick" (viscous) the fluid is, how quickly its velocity changes between layers, and how large the area of contact is.
It explains why honey (high viscosity) flows more slowly than water (low viscosity).
Force acts perpendicularly to the velocity ‘gradient’
Heat conduction
Type of physical transfer, where heat is transferred from one object to another of a different temperature.
How is the amount of heat energy transferred calculated?
Q = -k . dt/dx . S . t
Q - Heat energy transferred
k - thermal conductivity coefficient
dt/dx - thermal gradient
S - contacting area
What are the medical applications of thermal conduction & heat transfer?
Thermal therapy - warming pads and compresses, localised application of heat, dilation of blood vessels, increase blood flow to area of pain
Cryosurgery - application of extreme cold to impair or destroy cance
Transplantation
What is free surface of a liquid?
Outermost layer of a liquid in contact with air or another medium (e.g., water-air interface).
What is surface tension?
Molecules at the surface experience cohesive forces pulling inward, creating tension. This minimises surface area and leads to phenomena like spherical water droplets.
Formula for surface tension
Fsur = σ ⋅ I
σ - is equal to the amount of energy required for increase of the boundary surface
I -
What are surfactants?
Substances that lower surface tension
What is a surface acting substance (SAS)?
A chemical that alters the surface tension of a liquid at the interface between two phases (e.g., liquid-air, liquid-liquid, or liquid-solid)
Positive SAS: Decrease surface tension (e.g., detergents, soaps).
Negative SAS: Increase surface tension (e.g., sugar, salt, glucose).
What is additional pressure?
Refers to the extra pressure exerted due to the curvature of a liquid's surface at an interface, such as in bubbles, droplets, or liquid films.
This phenomenon arises because of surface tension, which acts to minimise the surface area of the liquid.
What is Laplace law?
Describes the relationship between the curvature of a surface and the pressure difference across that surface, particularly for curved liquid interfaces like bubbles, droplets, or blood vessels. It highlights how surface tension creates an additional pressure inside a curved surface.
Laplace explanation
When you blow air into the bubble, the surface of the bubble (the soapy water) stretches. This surface wants to shrink and be as small as possible because of something called surface tension. Surface tension acts like a "skin" on the bubble that pulls everything inward.
Now, here’s where Laplace’s Law comes in:
The smaller your bubble is, the harder the surface pulls (the pressure inside becomes bigger).
If you make the bubble bigger, the surface doesn’t pull as hard (the pressure inside becomes smaller).
What is the equation for Laplace law?
Δp= 2σ / r
Δp: The additional pressure inside the curved surface (Pa).
σ: The surface tension of the liquid (N/m).
r: The radius of the curvature of the surface (m).
What is an embolism?
Occurs when air bubbles enter blood vessels, the pressure difference on both sides of the bubble cause different curvatures on opposite sides of the bubble.
Low pressure - small radius
High pressure - greater radius
What is ideal fluid?
An ideal fluid is a theoretical concept used in fluid mechanics to simplify the analysis of fluid flow.
It is a fluid that has certain idealised properties that do not necessarily exist in real-world fluids.
These assumptions allow us to focus on the fundamental principles of fluid motion without the complexities introduced by real fluid behaviour.
What is a real fluid?
A real fluid is a fluid that exists in the physical world and exhibits properties that deviate from the assumptions of an ideal fluid.
Unlike ideal fluids, real fluids have viscosity, can be compressible, and may exhibit complex behaviours like turbulence, energy losses, and temperature-dependent changes.
What determines the type of flow?
Reynolds number, evaluated from the fluid properties.
Re < 2000 = Laminar
Transitional 2000 < Re < 4000
Re > 4000 = Turbulent
What is Reynolds number?
What is laminar flow?
Erythrocytes move in parallel layers
Low energy loss
No sound is generated
Flow is smooth and predictable
What is turbulent flow?
Fluid moves chaotically
Mixing of layers
Flow is irregular
Vortical motion
Sound generation
What conditions is turbulent motion observed in?
Stenosis, cardiac shunts, upper respiratory tract inflammation (air flow)
What is steady flow?
Steady flow type of fluid flow where the velocity of the fluid at any given point does not change over time.
This means that the fluid’s motion is consistent, and the properties of the flow, such as speed and direction, remain constant at each point in the flow field.
What is poiseuille law?
Poiseuille’s Law helps us understand how fluids flow through pipes. It says that the speed of flow depends on a few things:
the size of the pipe
the pressure difference
the thickness of the fluid
the length of the pipe
Size of pipe : wider pipe
wider the pipe, the faster the fluid flows
Pressure difference : bigger difference
The bigger the difference in pressure between the two ends of the pipe, the faster the fluid will move
The thickness of the fluid (viscosity):
Thicker fluids (like honey) move more slowly. Thinner fluids (like water) flow faster.
Length of the pipe :
The longer the pipe, the slower the fluid will flow because it has to travel farther.