Acid and Base Reactions
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
91 Terms
Hydrofluoric acid
HF - weak acid
Hydrochloric acid
HCl - strong acid
Nitrous acid
HNO2 - weak acid
Nitric acid
HNO3 - strong acid
Sulfurous acid
H2SO3 - weak acid
Sulfuric acid
H2SO4 - strong acid
Phosphoric acid
H3PO4 - weak acid
Carbonic acid
H2CO3 - weak acid
Acetic acid
CH3COOH - weak acid
citric acid
C6H8O7 - weak acid
Sodium hydroxide
NaOH - strong base
Barium hydroxide
Ba(OH)2 - strong base
Ammonia
NH3 - weak base
Ammonium
NH4+ - weak acid
two types of inorganic acid groups:
hydrohalic acids: hydrogen + halogen
oxyacids: containing oxygen atom/s
hydrohalic acid nomenclature:
prefix + suffix
hydro——ic acid
halogenic oxyacids nomenclature:
1 oxygen -
2 oxygen -
3 oxygen -
4 oxygen -
hypo — ous acid
— ous acid
— ic acid
per — ic aid
define acid
a substance whose molecules or ions are able to donate protons (hydrogen ions).
define base
a substance whose molecules or ions are able to accept protons (hydrogen ions)
acid + base →
salt + water
hydrochloric acid + sodium hydroxide
equation:
NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq)
neutralisation reactions are ____thermic::
exothermic
hydrochloric acid + barium hydroxide
equation:
2HCl(aq) + Ba(OH)2 (aq) → 2H2O + BaCl2 (aq)
acid + carbonate →
salt + water + carbon dioxide
hydrochloric acid + sodium carbonate
equation:
2HCl(aq) + Na2CO3 (aq) → H2O(l) + CO2 (g) + 2NaCl(aq)
sulphuric acid + copper carbonate
H2SO4 (aq) + CuCO3 (aq) → CuSO4 (aq) + H2O(l) + CO2 (g)
acid + metal →
salt + hydrogen gas
acid + metal oxide →
salt + water
sulphuric acid + magnesium
H2SO4 (aq) + Mg(s) → MgSO4 (s) + H2 (g)
equation for dissolution of an acid
acid → positive hydrogen + negative ion
equation for dissolution of vinegar
ethanoic acid → hydrogen ion + acetate ion
CH3COOH → H+ + CH3COO-
monoprotic acids
acids that donate one proton
hydrochloric acid HCl
nitric acid HNO3
ethanoic acid CH3COOH
amphoprotic substance
a substance that cab both accpet and donate a proton (H+)
polyprotic acids
acids that donate more than one proton
sulfuric acid H2SO4 - donate 2 protons - diprotic
carbonic acid H2CO3 - donate 2 protons - diprotic
phphosphoric acid H3PO4 - donate 3 protons - triprotic
dissociation of acids in water
splitting into positive and negative ions when dissolved in water
acids react faster if ____
there a more ions to react
acid strength refers ____
to the extent of dissociation of the acid
strong acids create _____
lots of hydrogen ions
strong acids react _____
easily
weak acids create _____
few or no hydrogen ions
weak acids react _____
poorly
strong acids
hydrochloric acid, HCl
sulfuric acid H2SO4
nitric acid, HNO3
perchloric acid, HClO4
hydrobromic acid, HBr
hydroiodic acid, HI
strong bases
sodium hydroxide, NaOH
potassium hydroxide, KOH
lithium hydroxide, LiOH
calcium hydroxide, Ca(OH)2
strontium hydroxide, Sr(OH)2
barium hydroxide, Ba(OH)2
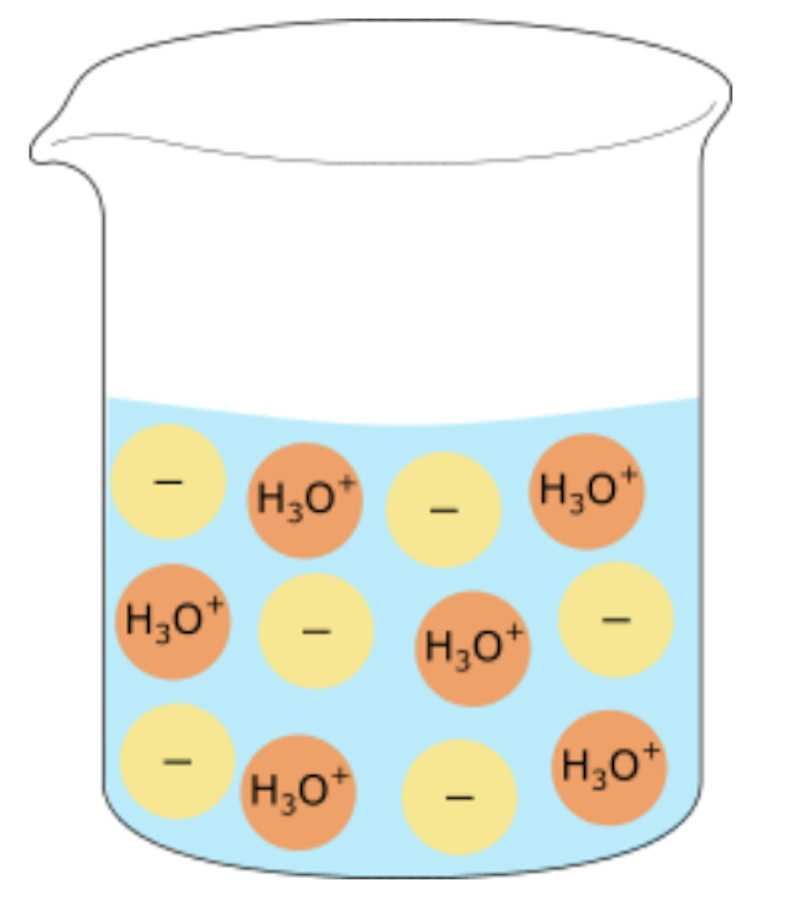
strong acid, concentrated solution
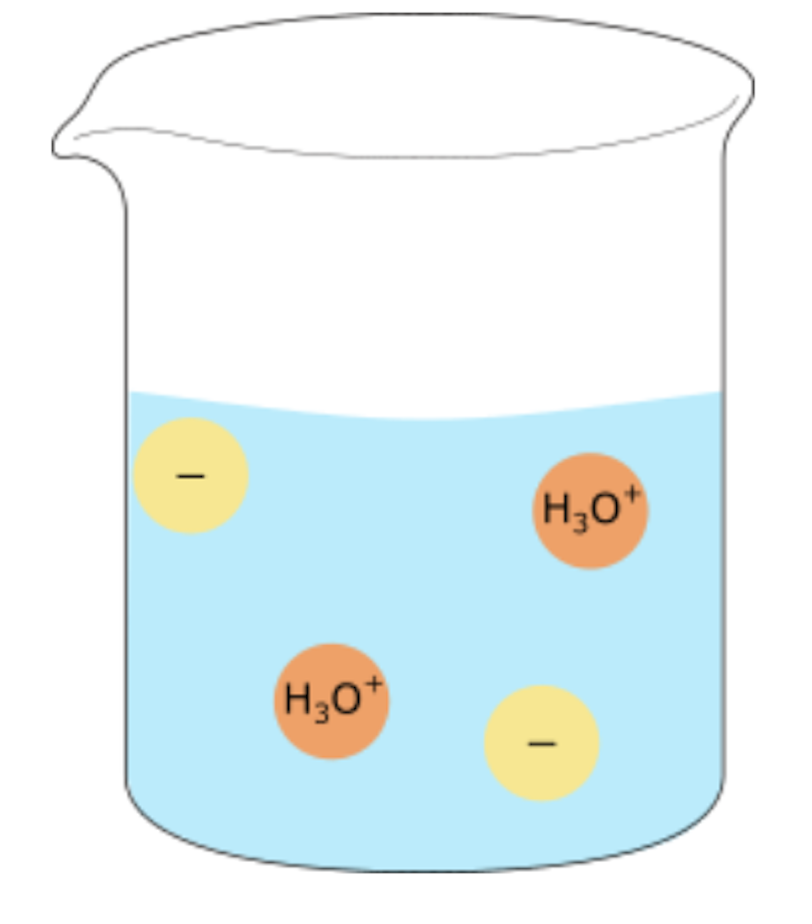
strong acid, dilute solution
A strong acid dissociates ____
almost fully in water and there will have high electrical conductivity as there are many ions present.
A weak acid dissociates ____
partially and therefore has less ions present, so has lower electrical conductivity.
acid concentration refers ____
to the number of moles present in a given volume.
chemical properties of acids
corrode materials
taste sour
conduct electricity in solution
react easily
chemical properties of bases
caustic
slippery
taste bitter
general formula for acid dissociation in water:
HA + H2O ⇌ H3O+ + A-
strong acids: reaction completion
generally reversible
however some acids donate all hydrogen ions that the reaction is irreversible / gone to completion.
equilibrium lies very much to the right
weak acids: reaction completion
do not go to completion (partially ionise)
reversible ∴ establishes an equilibrium
reverse reaction is favoured (ions react very easily to reform reactants)
strong acid: pH
pH ≈ 1
strong acid: pKa
pKa ≈ 0
weak acid: pH
pH = 2-6
weak acid: pKa
pKa > 3
strong base: pH
pH ≈ 14
strong base: pKb
pKb = low (0 - 3)
weak base: pH
pH = 8-11
weak base: pKb
pKb > 3
pH:
is a measure of the concentration of hydrogen ions in a solution.
pOH:
is a measure of the concentration of hydoxide ions in a solution.
pH equation:
pH = -log [ H+]
[H+] =
10-pH
pOH equation:
pOH = -log [OH-]
[OH-] =
10-pOH
relationship between pH and pOH
pH + pOH = 14
Pure Water pH:
pH = 7
[OH-] = [H3O+] = 10-7 M
Acid Dissociation Constant:
Ka - the equilibrium constant describing the extent of dissociation fro a weak acid. Ka is measured in mol/L.
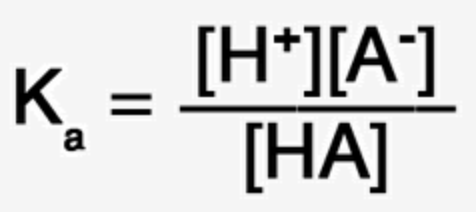
conjugate acid-base pairs refer to ____
two substances that differ only by the presence of a proton (H⁺)
Conjugate Acid:
A species formed by adding a proton to a Bronsted-Lowry base e.g. NH4+ from NH3.
Conjugate Base:
A species formed by the loss of a proton from a Bronsted-Lowry acid e.g. Ch3COO- from CH3COOH.
Acidic Buffer:
A buffer solution comprising a weak acid and its conjugate base, often in the form of a salt, such as a mixture of ethanoic acid (CH₃COOH) and sodium acetate (CH₃COONa).
Basic Buffer:
A buffer solution composed of a weak base and its conjugate acid, often in the form of a salt.
Buffer Solution:
A solution capable of resisting pH changes when small amounts of acid or base are added.
Lavoisier theory:
description:
acids were substances that contained oxygen atoms
experimented on oxides of non-metals
limitations:
many acids do not contain oxygen
e.g. HCl
Davy theory:
description:
acids contained replaceable hydrogen atoms
HCl did not contain oxygen whilst still acting as an acid
limitations:
no explanation as to when, or how the molecules interacted
Arrhenius theory:
description:
acids ionise in water to produce hydrogen ions (H+) and bases ionise in water to produce hydroxide ions (OH-).
limitations:
only works for acids and bases in aqueous solutions
some acids and bases operate without being in solution
Bronsted-Lowry theory:
description:
acids are proton donors
bases are proton acceptors
limitations:
theory requires hydrogen atoms to be present within a molecule to be classified as an acid or a base
molecules e.g. BF3 acts an acid
primary purposes of titrations:
quantitive analysis
identification of unknown substances
quality control
PSS
pure soluble stable (primary standard solutions)
primary standard solution:
a solution that has an accuratrly known concentration.
preparation of primary standard solution: summary
Measure
Transfer
Ensure transfer of all
Swirl to dissolve
Fill and invert to mix
Phenolphthalein
Colourless → Pink
8.3 - 10.0
weak acid + strong base
Bromothymol Blue
Yellow → Green
6.0 - 7.6
strong acid + strong base
Phenol Red
Yellow → Red
6.8 - 8.0
strong acid + strong base
Methyl Red
Red → Yellow
4.4 - 6.2
strong acid + weak base
Methyl Orange
Red → Yellow
3.2 - 4.4
strong acid + weak base
applications of neutralisation reactions in everyday life:
vinegar to treat wasp or jelly fish stings:
toxins are alkaline (basic)
vinegar contains acetic acid (weak acid)
toothpaste:
contains various bases
sodium hydroxide NaOH
calcium carbonate CaCO3
alkalinity of toothpaste helps to neutralise acids produced by bacteria in the mouth
applications of neutralisation reactions in chemical industry:
chemical spills:
bases used to neutralise acid spills
acids used to neutralise base spills
generally weak acids and bases as strong acid + strong base reaction is highly exothermic.