Year 10 GCSE Chemistry - C2.1 Bonding
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
What electrons do
Orbit the nucleus in shells
First shell holds a maximum of -
2 electrons
Second shell holds a maximum of -
8 electrons
Third shell holds a maximum of -
8 electrons
What the group # shows (periodic table)
# outer electrons
What the period # shows (periodic table)
# electron shells
Properties of noble gases
Inert and stable
Ion
Electrically charged particle formed when an atom, or group of atoms, loses or gains electrons
Metal atoms do what to form + ions
Lose electrons
Non-metal atoms do what to form - ions
Gain electrons
What stay constant when an atom forms an ion
# protons and neutrons
What you need to work out an ion's electronic structure
Electronic structure of the original ion, and the # electrons it lost or gained
Electronic structure of a sodium atom
2.8.1
Stable atoms or ions have -
Full outer shells
Electron diagram
Represents the electronic structure of an atom or ion
How you draw an electron diagram
A circle to represent each shell, and dots or crosses to represent its electrons. Ions go inside brackets with the change written at the top right. The element's symbol potentially written at the centres instead of showing a nucleus. Have a go at a few
Dot-and-cross diagrams
Use of both dots and crosses allow you to see which atoms provided particular electrons
What dot-and-cross diagrams can be used to show
Covalent bonds
What happens when a metal and non-metal react
Electrons are transferred from the metal atoms to non-metal atoms so both achieve more stable electronic structures
What ionic compounds contain in their solid state
Positive and negative ions arranged in a regular way, called a giant ionic lattice
Ionic bonds
Strong, electrostatic forces of attraction between metal and non-metal atoms, holding oppositely-charged ions in place, acting in all directions
Space-filling model
Way of representing ionic compounds
Binary ionic compound
Composed of two elements (a single metal e.g. sodium and non-metal e.g. chlorine)
Giant ionic lattice
Exists in three dimensions, but you can only draw it in two dimensions
What balls and sticks represent in a ball-and-stick model
Balls represent ions and plastic links represent an ionic bonds
Positive things about ball-and-stick models
Give you a clearer idea of the structure and shape of the lattice
Limitations of ball-and-stick models
Ions should be close together - bonds are forces rather than physical objects made from matter
What you can write before brackets in ions notation
Coefficient
What we need to know to find formulae of ionic compounds
Charges of ions
How we can find charges of ions
Periodic table
Transition metals form -
More than one ion
Positive (group) ions
Ammonium, NH4+
Negative (group) ions
Hydroxide, OH -, nitrate, NO3 -, sulfate, SO4 2- and carbonate, CO3 2-
Covalent bond
Shared pair of electrons, forming between two non-metal atoms when they get close enough to share atoms in their outer shells. They therefore complete their outer shells
What each pair of electrons in the intersection of overlapping circles of a dot-and-cross diagram represents
A covalent bond
When drawing a dot-and-cross diagram for covalent bonds, only the what are shown?
Outer shells
Stick notation
Each line between symbols represents a bond. You can have multiple lines to represent multiple e.g. double bonds
Molecule
Particle in which non-metal atoms are joined to each other by covalent bonds
Simple molecule
Only contains a few atoms
Examples of simple molecules
Hydrogen, oxygen, water and carbon dioxide
Covalent bonds between atoms in a simple molecule are -
Strong
Intermolecular forces in a simple molecule are -
Weak
Intermolecular forces
Between molecules
Limitations of ball-and-stick models (for simple molecules)
Sizes of atoms and lengths of bonds are exaggerated, and it suggests that electrons that make bonds do not move
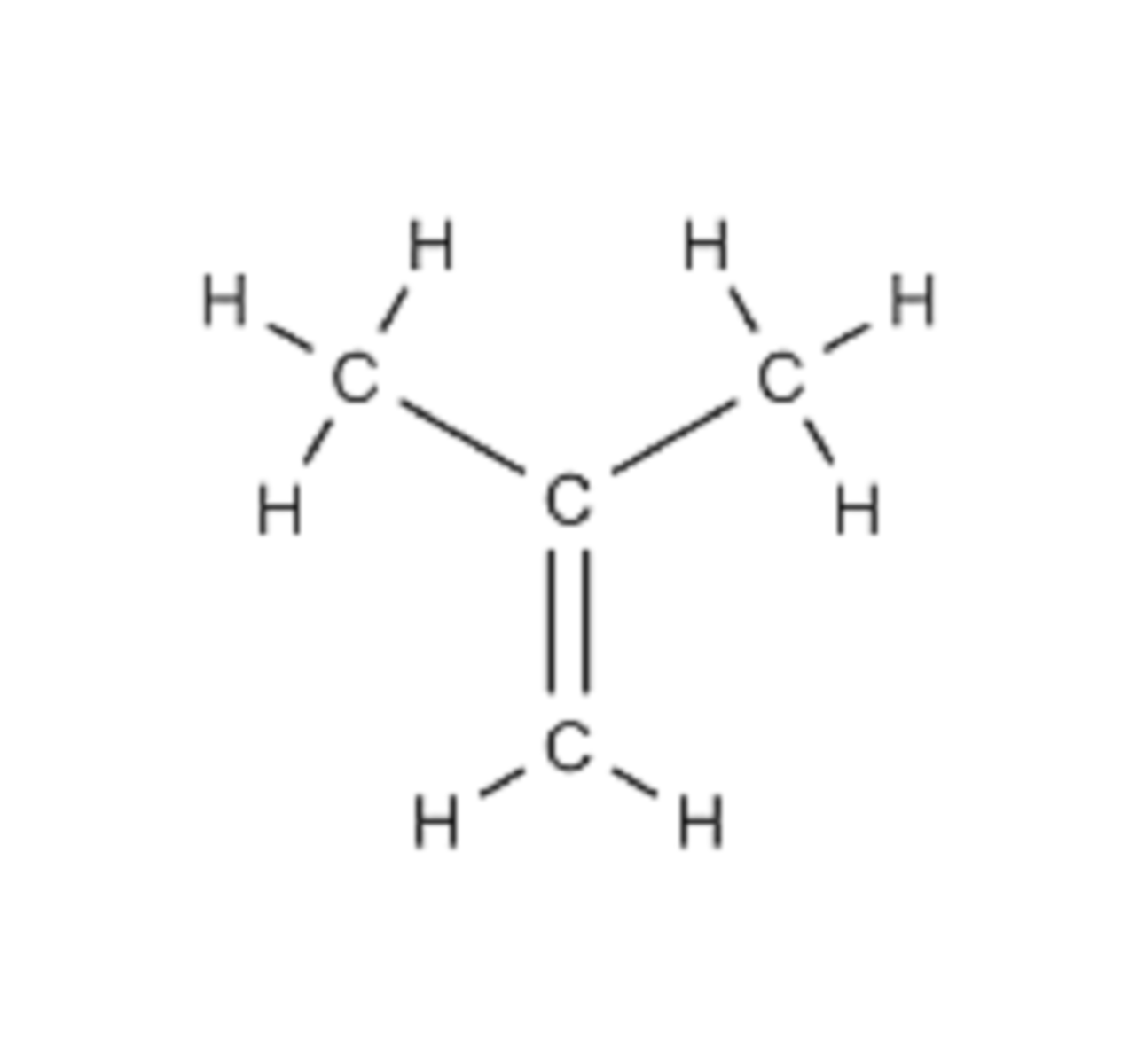
Displayed formula
Drawn for simple molecules, where each atom is represented by its chemical symbol and each covalent bond is a straight line
Limitations of a displayed formula
Does not show three-dimensional shape of molecule
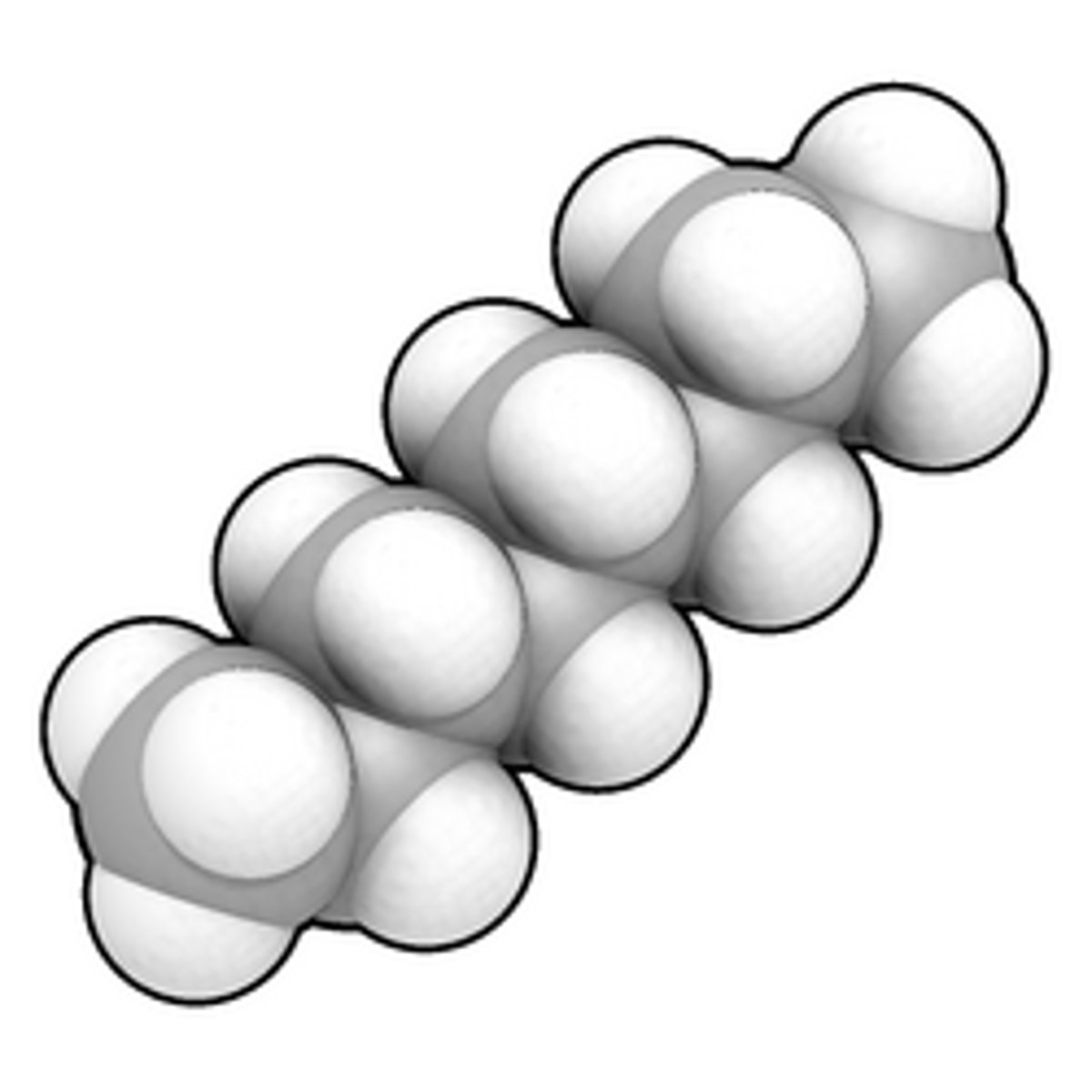
Space-filling models
Way of representing molecules, using interconnected spheres to show electron clouds of atoms connecting together
Giant covalent structures / lattices
Have a repeating lattice. Composed of very many non-metal atoms covalently bonded, arranged in a repeating, regular pattern called a giant lattice
Allotropes
Different forms of the same element in same physical state, having different molecular structures
Two examples of minerals that are allotropes of carbon
Diamond and graphite
What explains the differences between diamond and graphite
Arrangement of atoms
Empirical formula
Shows simplest whole-number ratio of elements in a compound
Empirical formula for diamond
C
Properties of diamond
Strong and hard, crystalline and high melting and boiling points
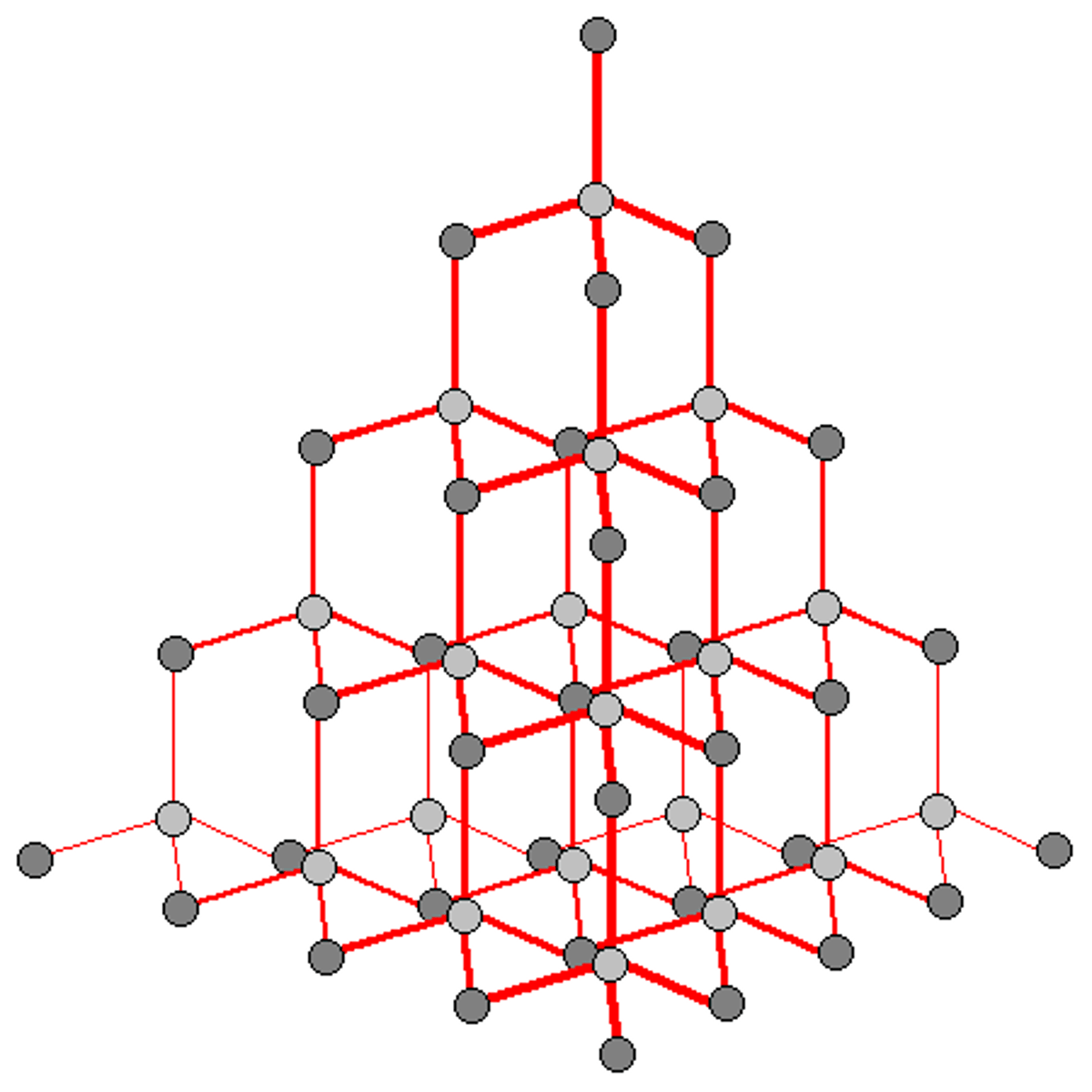
Crystalline
Solid made up of crystals in which particles are arranged in a regular, repeating pattern
Why diamond is crystalline
Each carbon atoms is covalently bonded to four other carbon atoms
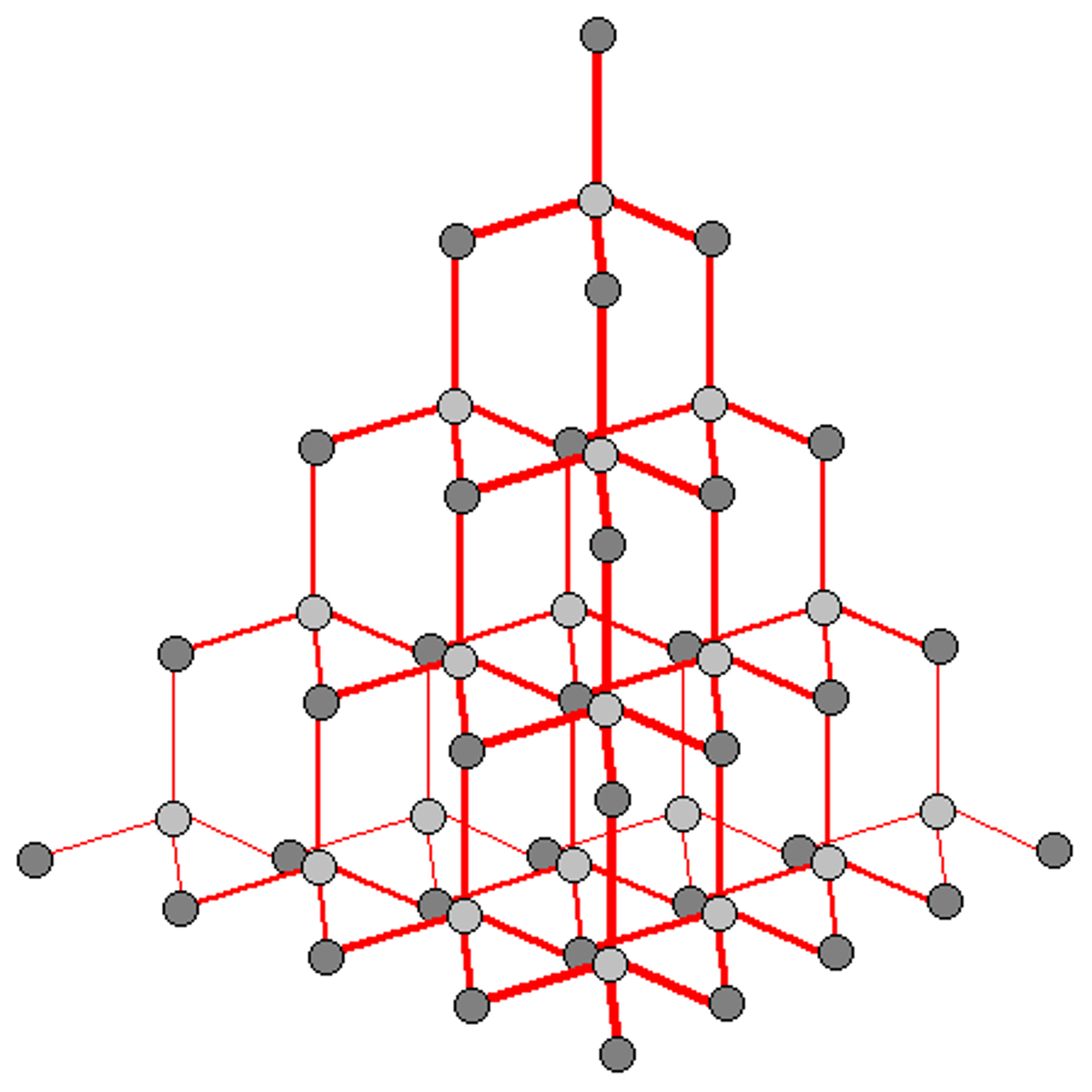
Structure of graphite
Each carbon atom is bonded to three others, its fourth unshared electron is delocalised. Has layers of covalently bonded carbon hexagon rings, although no covalent bonds between layers so can 'slide'
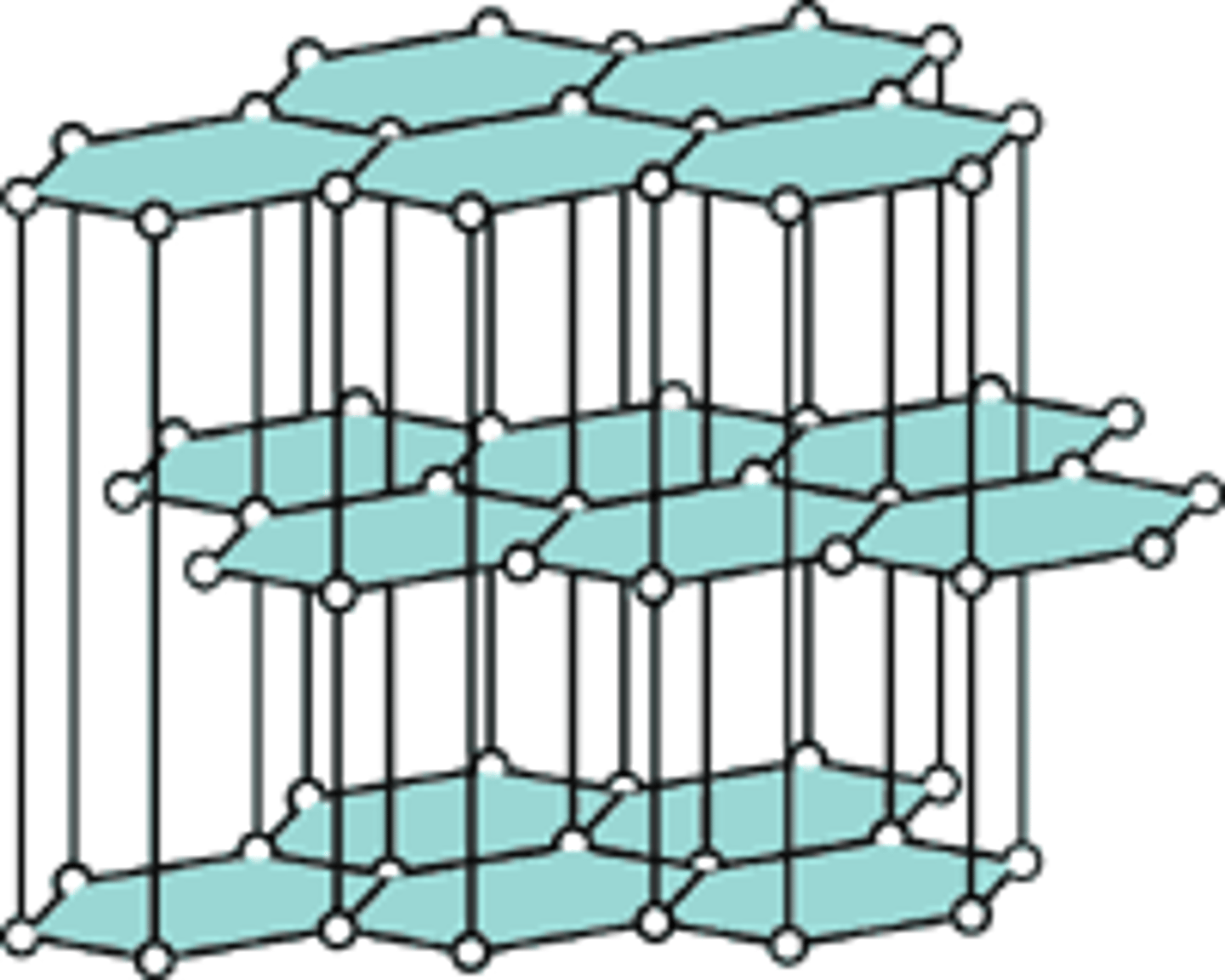
Properties of graphite
Soft and slippery, conducts electricity (delocalised electrons) and high melting and boiling points
Buckminster fullerene aka
Bucky ball
Properties of Buckminster fullerene
Cannot conduct electricity or heat
Structure of Buckminster fullerene
Allotrope of carbon with simple molecular structure, and so chemical formula C60
Fullerenes
Form of carbon having a large spheroidal molecule consisting of a hollow cage of sixty or more atoms, of which buckminsterfullerene was the first known example
What simple molecular substances are made of
Molecules
Properties of simple molecular substances
Low melting and boiling parts, as there are weak forces between molecules. Do not conduct electricity because molecules are not electrically charged - they are insulators
What atoms are held together by in covalent bonds
Shared electrons
State of metals, apart from mercury, at room temperature
Solid
Structure of metals
Atoms packed together in a regular way forming a giant metallic lattice
How a giant metallic lattice can be modelled
Drawing circles or spheres arranged in a regular pattern, in contact with each other
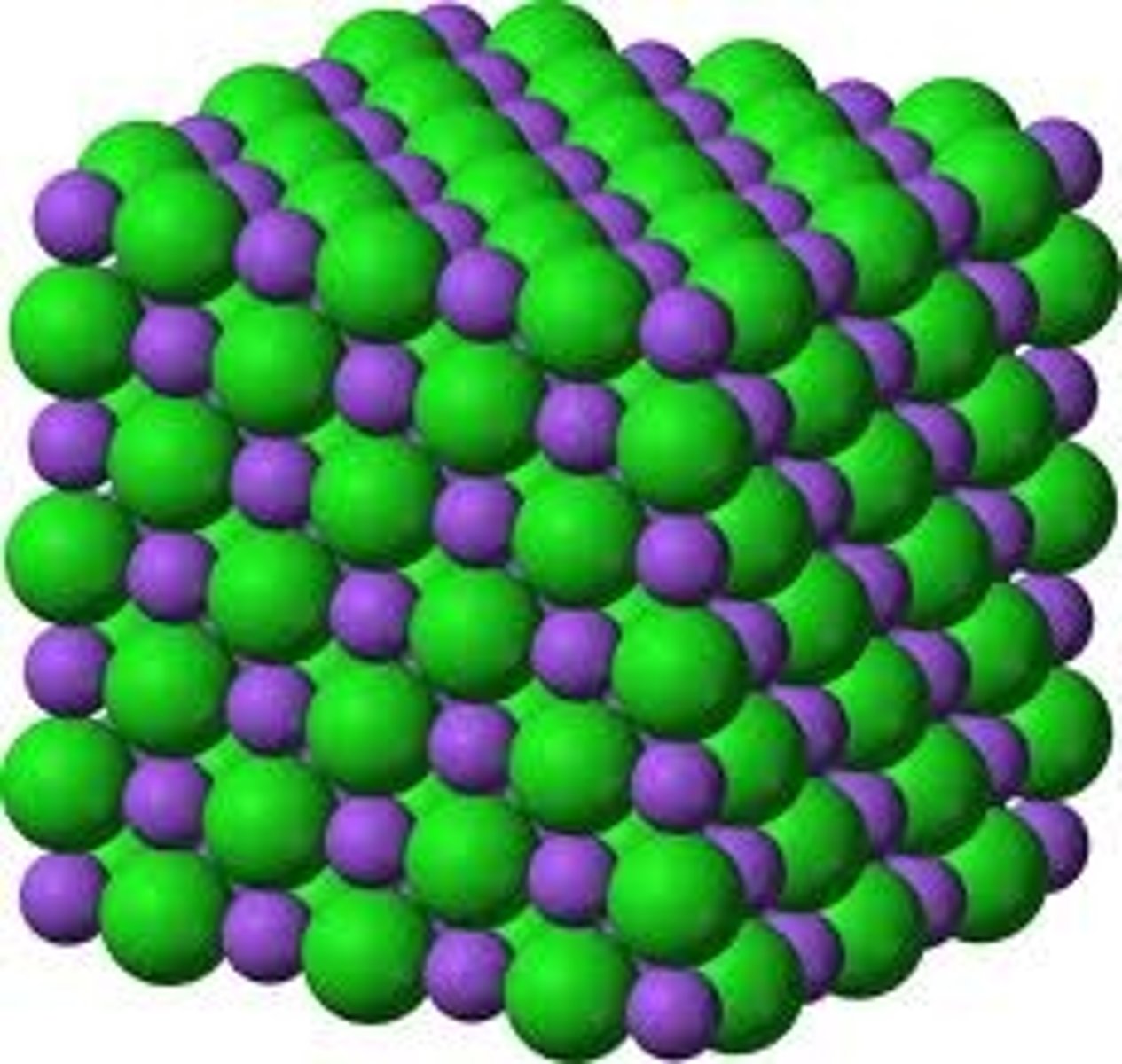
Properties of metals (remember, they link to structure!)
Conduct electricity, high melting and boiling points, shiny, malleable and ductile

Leading up to / formation of metallic bonds
Electrons leave outer shells of metal atoms forming s 'sea' of delocalised electrons around positively charged metal ions
Delocalised electrons
Free to move through the structure of a metal
Metallic bonds
Strong electrostatic forces of attraction between delocalised electrons and closely packed, positively charged metal ions
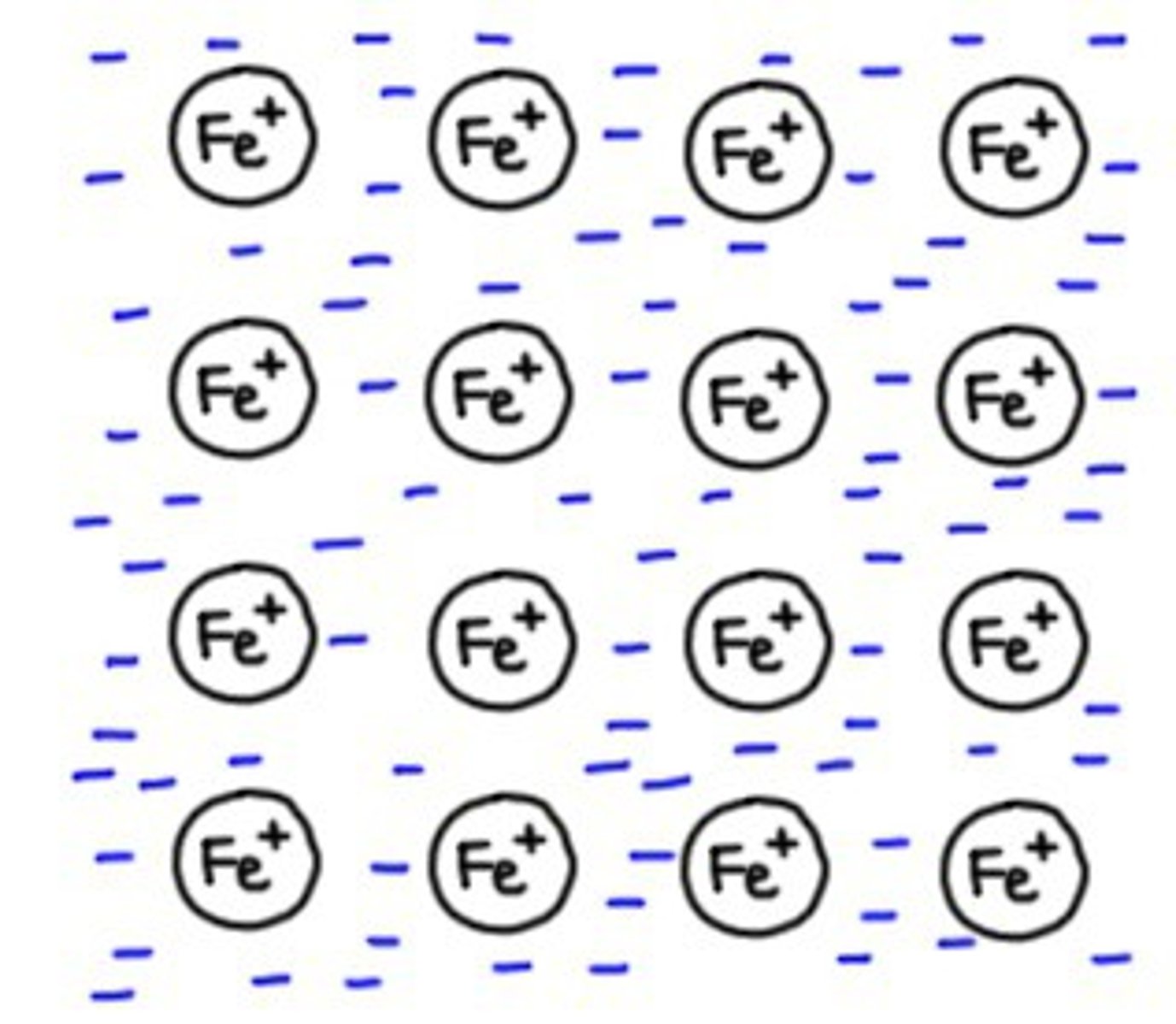
Models of metals
As a metallic structure extends in three dimensions, and its metallic bonding also extends in three dimensions, you lose some information when representing structure and bonding in two dimensions
Polymer (molecule)
Made from many smaller monomer molecules
Monomer
Simple molecule consisting of covalently-bonded, non-metal atoms, able to join end to end in chemical reactions to produces longer polymer molecules
Example of artificial polymers
Plastics such as nylon
Example of natural polymers in the body
Complex carbohydrates, DNA and proteins in skin
Many ethene molecules make
Polyethene / polythene
Many amino acids make
Protein e.g. keratin in hair
Simple model showing how polymers form
Squares or circles (representing monomers) might be joined up with a line to show a polymer
Three ways of modelling monomers
Dot-and-cross diagrams, space-filling models and ball-and-stick models
What is much more difficult for polymer molecules than monomers
Modelling them
Space-filling model DNA, reiterating difficulty of modelling polymers
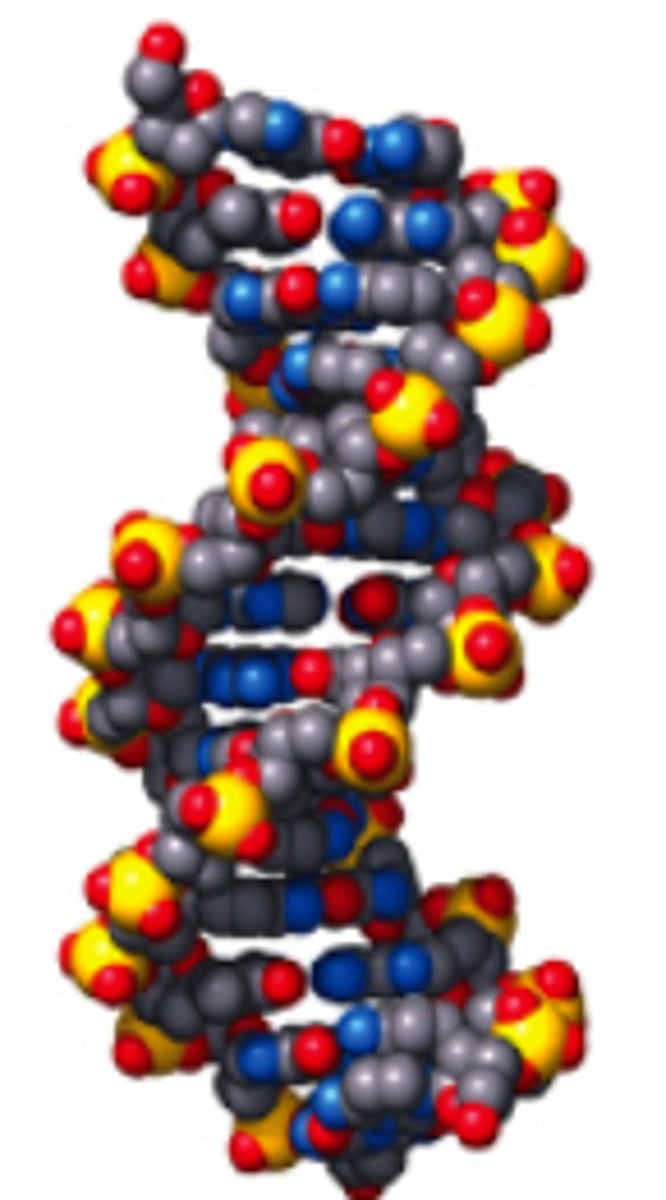
Starch
Complex carbohydrate
'Wavy-line' diagram for modelling polymers
Each polymer molecule is drawn as a wavy line, sometimes with straight lines in between them to represent covalent bonds between individual polymer molecules. Weak intermolecular forces between polymer molecules are not shown
Two examples of polymers made from several different types of monomers
Proteins and DNA
Example of polymer made from one type of monomer
Polythene
Formula for ethene
C2H4
What idea poly(ethene) can be modelled using
Repeating unit
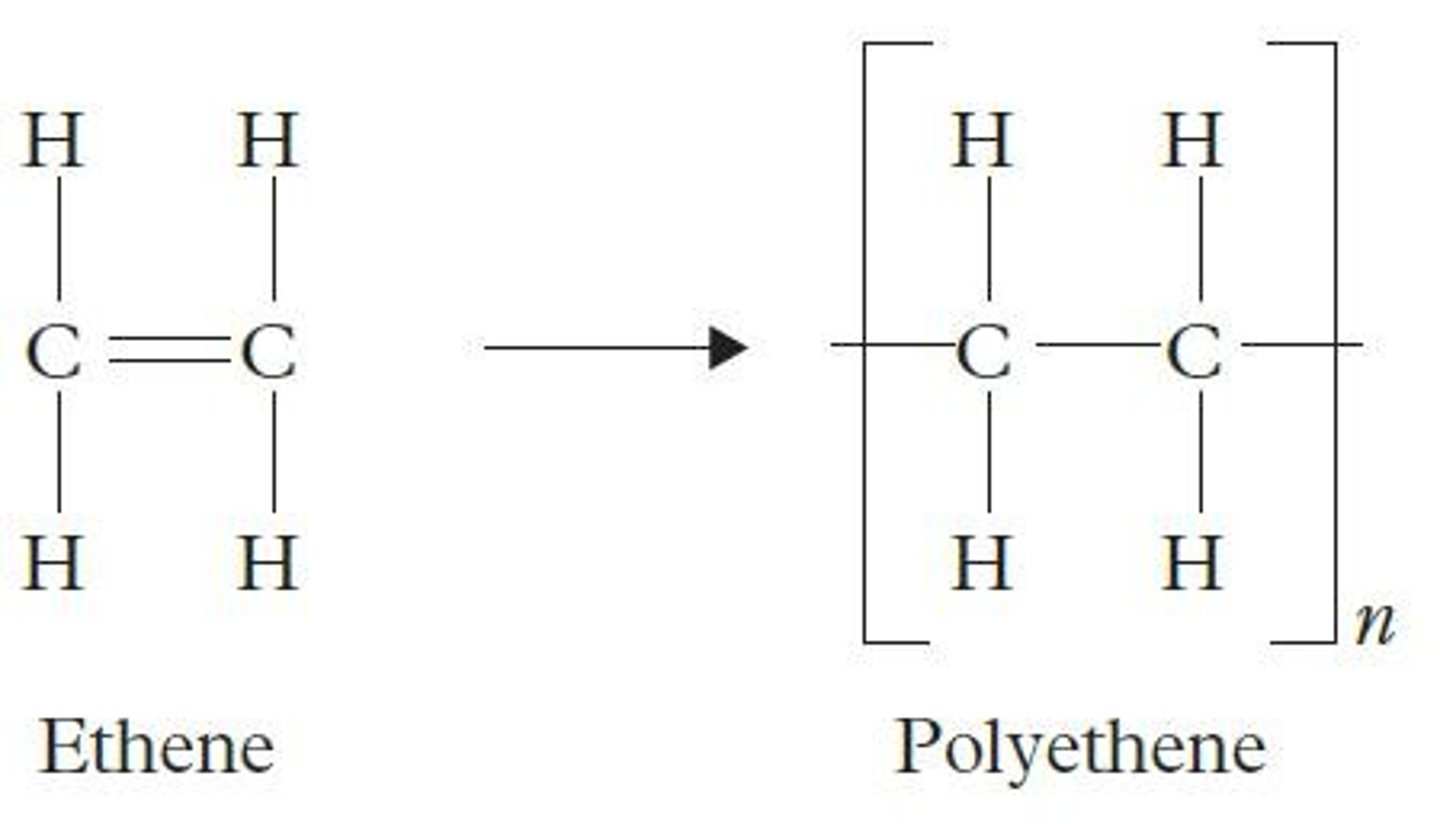
Repeating unit
Section of polymer repeated over and over again
Ionic bonding
Between metals and non-metals, where electron
Description of ionic bonding
Electrons are transferred from metal to non-metal atom. Results in giant ionic lattice
Properties of giant ionic lattices
Hard, crystalline, soluble in water and do not conduct when solid although do conduct when liquid or dissolved
What is required for a substance to conduct electricity
Charged particle free to move around
Why a metal can conduct electricity
Delocalised electrons are always free to move around
Why ionic compounds in their solid state cannot conduct electricity
Oppositely charged ions are fixed into place, unable to move around and carry a charge
Why ionic compounds dissolved in water can conduct electricity
Ions aren't held together by strong electrostatic forces of attraction anymore, therefore free to move around and conduct electricity