Chapter 4: Compounds & Stoichiometry (7%)
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
molecular weight
Molecular Weight vs. Formula Weight vs. Molar Mass
The sum of all the atomic weights of all the atoms in a molecule
Measured in amu/molecule
formula weight
Molecular Weight vs. Formula Weight vs. Molar Mass
The sum of all the atomic weights of all the ions in an ionic compound according to its empirical formula
Measured in amu/molecule
Numerically the same as molar mass but used in different applications
molar mass
Molecular Weight vs. Formula Weight vs. Molar Mass
The mass of one mole of a compound
Usually measured in g/mol
Numerically the same as formula weight but used in different applications
Avogadro’s Number
Represents the exact number of particles (atoms, molecules, ions, or electrons) contained in one mole of any substance
one mole
Avogadro’s number of particles = ____ ____ of a compound
6.022 × 10^-23 (particles)
Avogadro’s Number
molecular/formula weight
One mole of a compound has a mass in grams equal to the ____________/__________ _________ of the compound in amu
equivalents
What does this question describe:
“How many moles of the thing we are interested in will one mole of a given compound produce?”
3
How many equivalents of hydrogen does H3PO4 have?
1
How many equivalents of hydrogen does HCl have?
gram equivalent weight
The amount of a compound that produces one equivalent of the particle of interest, measured in grams

normality

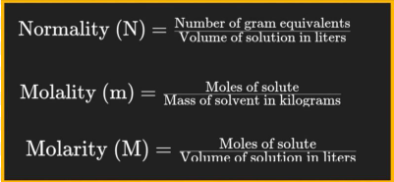
molarity, M, molarity, M, mole

Normality (N) is related to __________ (___) by multiplying the __________ (___) by the number of equivalents per _____ of compound
N = n x M
(n = the # of dissociable protons H+ / molecule of acid)
Molarity & Normality
Write out the relationship between Normality (N) and Molarity (M)
hydrogen, 1, H+, 1, H+
Normality
A 1 N solution of acid contains a concentration of ____________ ions = to ___ mole(s) of ____ per liter of solution
This is because a 1 N solution contains exactly ___ equivalent(s) of ____ per liter of solution
hydrogen, 2, H+, 2, H+
Normality
A 2 N solution of acid contains a concentration of ____________ ions = to ___ mole(s) of ____ per liter of solution
This is because a 2 N solution contains exactly ___ equivalent(s) of ____ per liter of solution
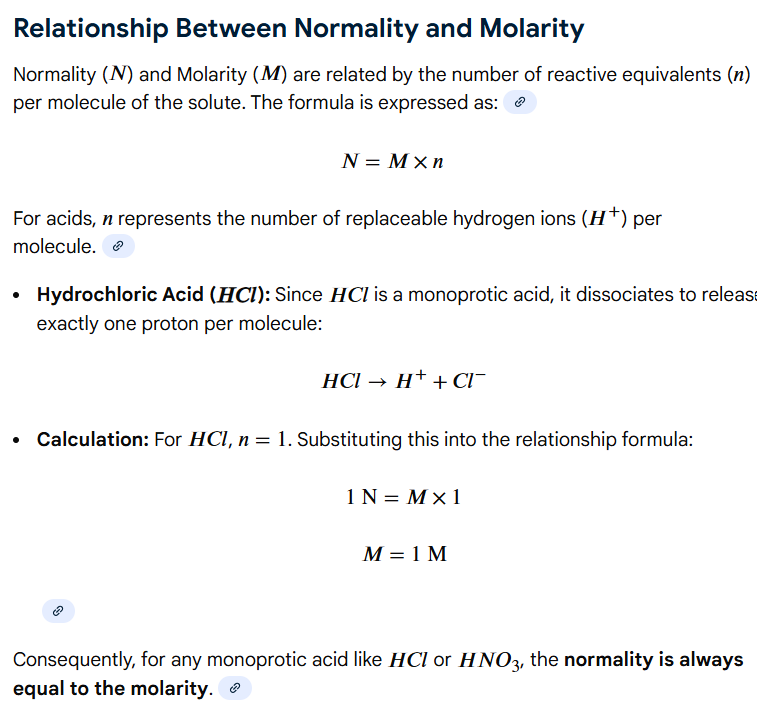
1 M, 1
(HCl is monoprotic, so 1x equivalent of base are required to neutralize the single proton of the acid)

Molarity & Normality
What is the molarity of HCl in a 1 N HCl solution?
How many equivalents of base are needed to neutralize this acid?
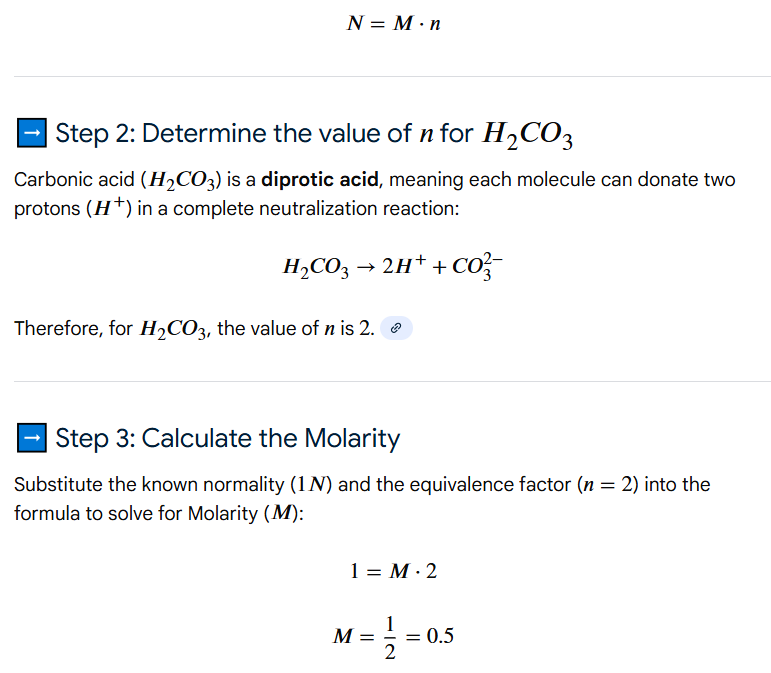
0.5 M, 2
(H2CO3 is diprotic, so 2x equivalents of base are required to neutralize both protons of the acid)

Molarity & Normality
What is the molarity of H2CO3 in a 1N H2CO3 solution?
How many equivalents of base are needed to neutralize this acid?
equivalents
The amount of a substance (in moles) that reacts with, combines with, or is equal to one mole of another substance
Often defined by the number of electrons or hydrogen ions it donates or accepts in a reaction, essentially representing its reactive capacity.
Moles of the species of interest
acid-base, oxidation-reduction

the 2 types of reactions where equivalents are most often seen
Law of Constant Composition
Law stating that any sample of a PURE chemical compound ALWAYS contains the SAME ELEMENTS in the SAME FIXED RATIO by MASS, regardless of its source or preparation method
empirical formula
Empirical Formula vs. Molecular Formula
The smallest whole-number ratio of the elements in a compound
EX: CH
Ionic compounds ONLY have this formula type
molecular formula
Empirical Formula vs. Molecular Formula
Either the same as or a multiple of the other formula
Gives the exact number of atoms of each element in a compound
EX: C6H6

C3H4O3, C9H12O9

Empirical & Molecular Formulas
What are the EMPIRICAL & MOLECULAR formulas of a carbohydrate that contains 40.9% carbon, 4.58% hydrogen, and 54.52% oxygen & has a molar mass of 264 g/mol? (pg. 131)
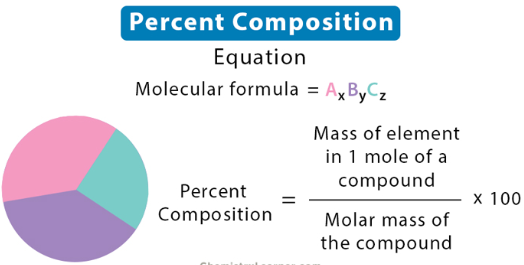
percent composition

The percent of a specific compound that is made up of a given element
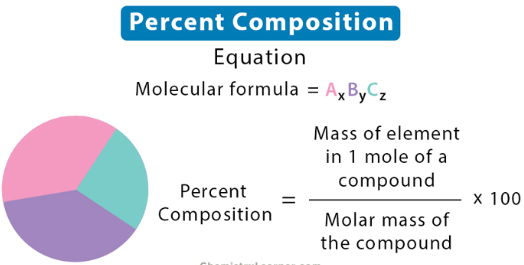
(mass of individual element / molar mass of compound) x 100

Percent Composition
Write out the formula for calculating the percent composition by mass of an element in a compound
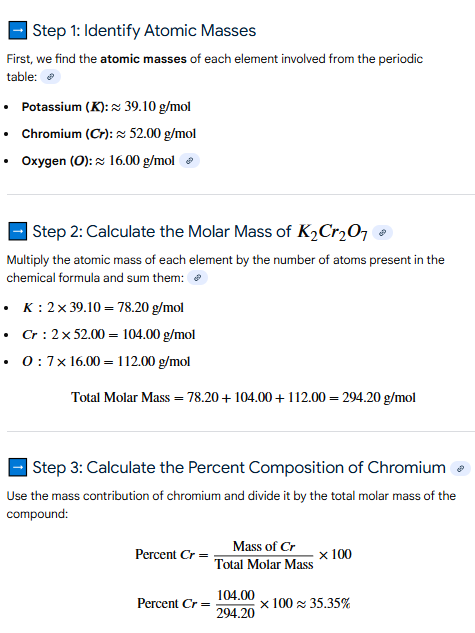
33%

Percent Composition
What is the approximate percent composition of chromium in K2Cr2O7? (pg. 130)
combination
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When two or more reactants combine to form one product
A+B→C
decomposition
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When one reactant is chemically broken down into two or more products
A→B+C
combustion
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When a fuel and an oxidant (typically oxygen) react, forming the products water and carbon dioxide (if the fuel is a hydrocarbon)
Fuel + O2 → CO2 + H2O + Energy (heat/light)
single-displacement
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When an ion of one compound is replaced by an atom/ion of another element
Often further classified as oxidation-reduction reactions
A + BC → AC + B
double-displacement
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When elements from two different compounds trade places with each other to form two new compounds
Occurs when one of the products is removed from the solution as a precipitate or gas OR when two of the original species combine to form a weak electrolyte that remains undissolved in solution
Usually happen in water
Often result in a visible change, such as the formation of a solid precipitate, a gas, or water
AB + CD → AD + CB
neutralization
Types of Reactions
WORD BANK: Combination, Decomposition, Neutralization, Combustion, Single-Displacement, Double-Displacement
——
When an acid reacts with a base to form a salt (and usually water)
H+ + OH- → H2O + salt
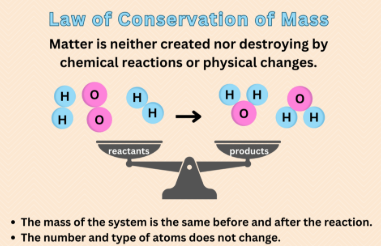
Law of Conservation of Mass

Law stating that the # of atoms of each element on the reactant side MUST EQUAL the # of atoms of that element on the product side
balancing the least common atoms, balancing the more common atoms, balancing charge (if necessary)
list in order the 3 steps in balancing chemical equations
convert from the given units to moles, use the mole ratio, convert from moles to the desired units
list in order the 3 steps used in stoichiometry/dimensional analysis problems
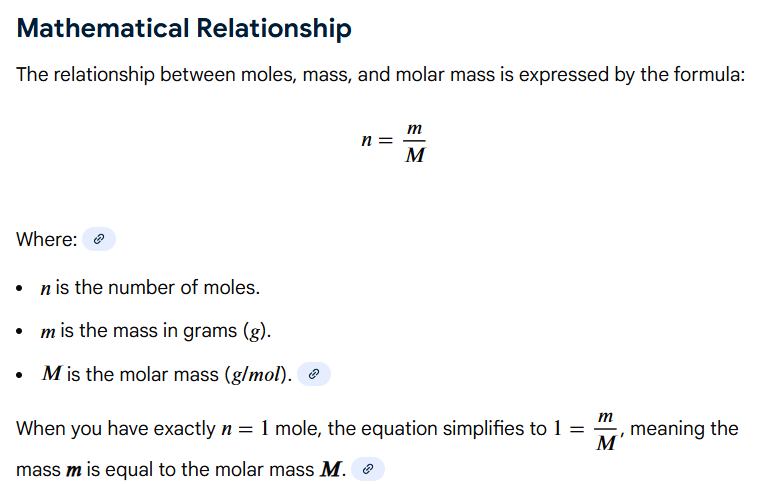
its molar mass in grams (from the periodic table)
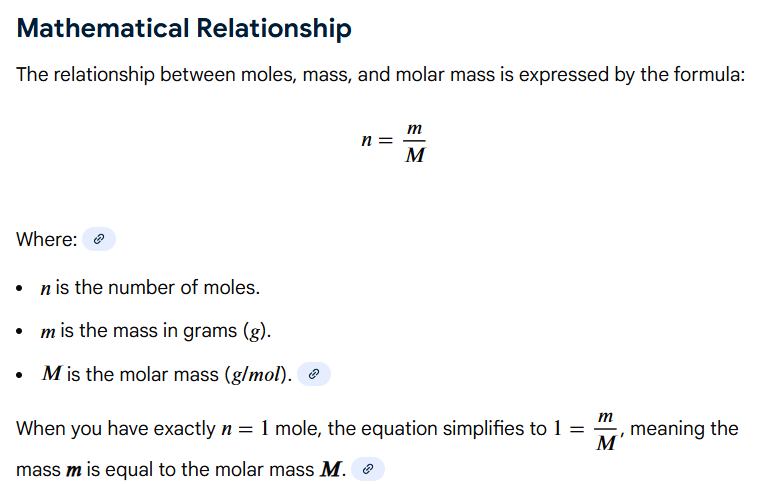
1 mole of any substance = ?
22.4
1 mole of any ideal gas at standard temp & pressure (STP) = ___ L
limiting reagent
The reactant that will be consumed first (gets completely consumed) in a chemical reaction
Determines & limits the maximum amount of product that can be formed
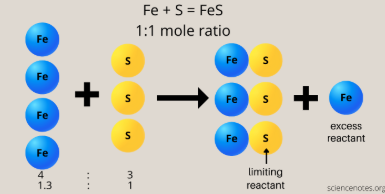
excess reagents
the other reactants present in a chemical reaction aside from the limiting reagent
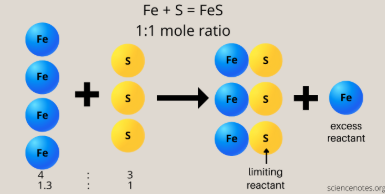
theoretical yield
the max amount of product that can be formed in a chemical reaction, as predicted from the balanced equation, assuming that all of the limiting reactant is consumed with no side reactions
actual yield
the amount of product one actually obtains during a reaction
actual yield
Which is typically LOWER: theoretical yield or actual yield?
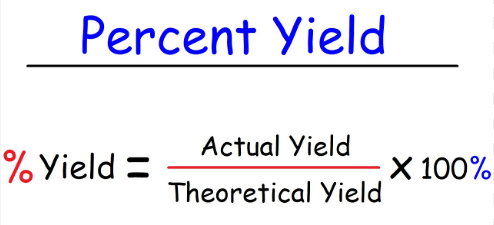
(actual yield / theoretical yield) x 100

Write out the formula for calculating the percent yield
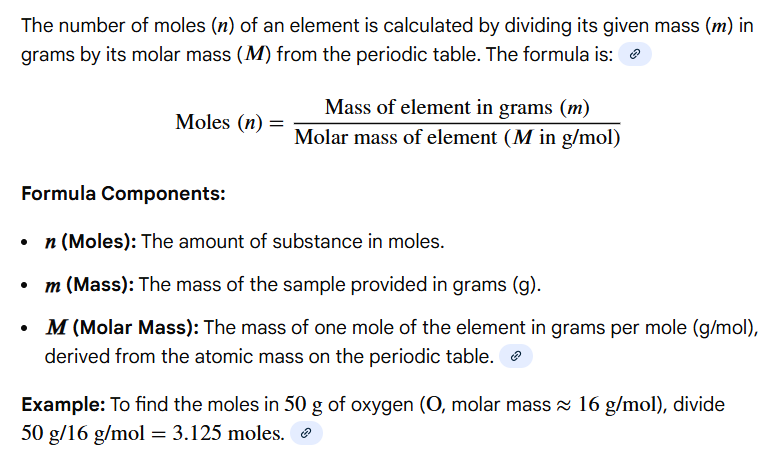
mass of a sample (m) / molar mass (MM)

Write out the formula for calculating the # of moles of an element in a sample
Variables: mass of a sample (m), molar mass (MM)

GEW = molar mass of compound (MM) / number of particles of interest produced or consumed per molecule of compound (n)

Write out the formula for calculating the Gram Equivalent Weight of a compound
Variables: molar mass of compound (MM), number of particles of interest produced or consumed per molecule of compound (n)
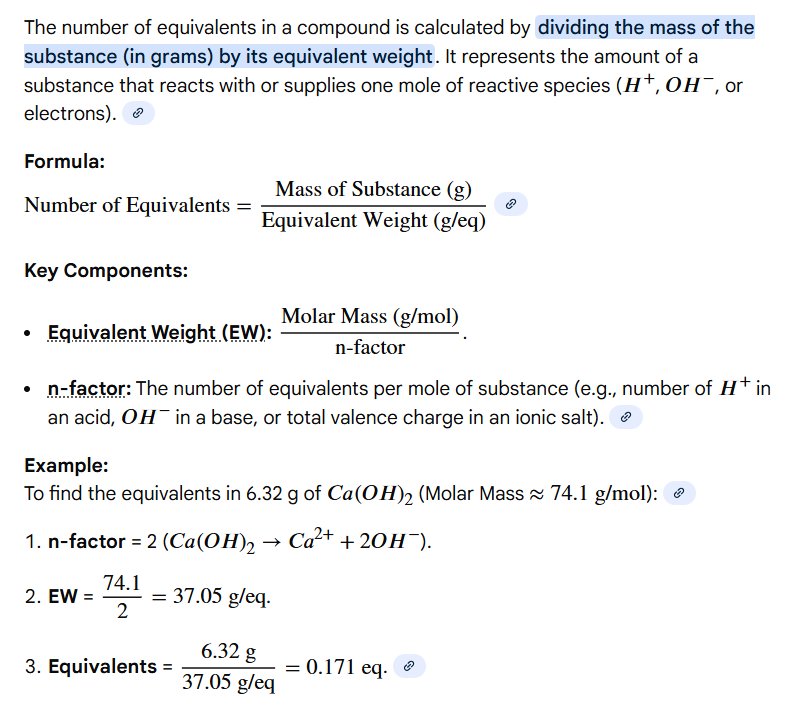
mass of compound (m) / gram equivalent weight
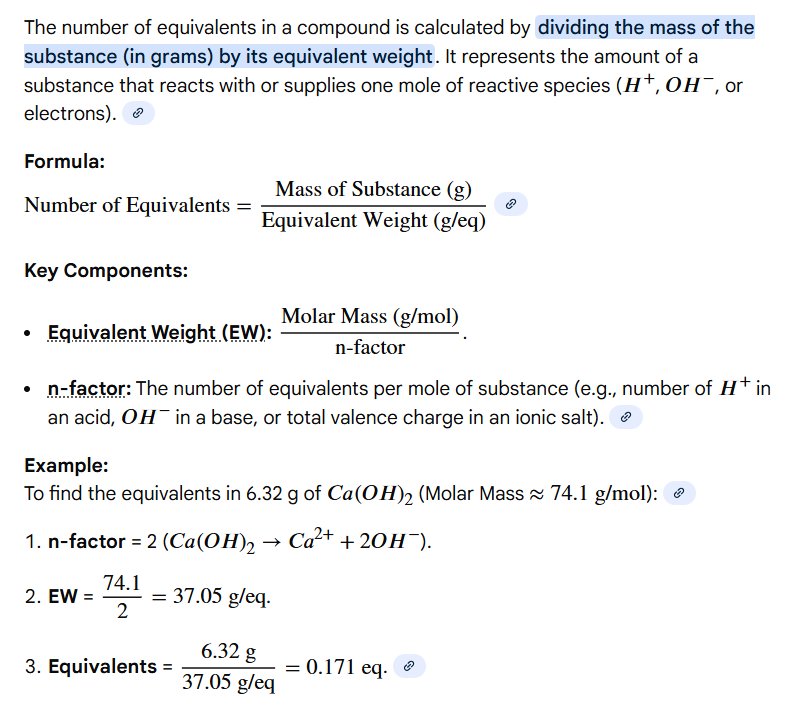
Write out the formula for calculating the # of equivalents present in a compound
Variables: mass of compound (m), gram equivalent weight
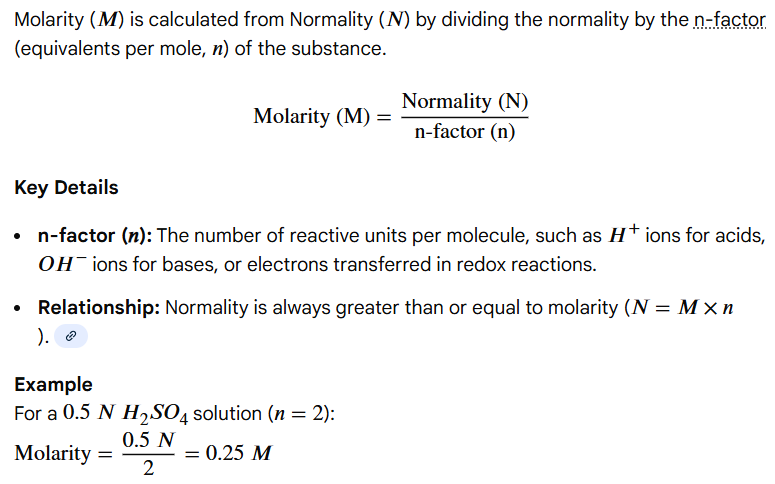
Molarity (M) = Normality (N) / n
(n = # of protons, hydroxide ions, electrons, or ions produced or consumed by the solute)
Write out the formula for calculating molarity (M) from normality (N)
Variables: normality (N), of protons, hydroxide ions, electrons, or ions produced or consumed by the solute (n)
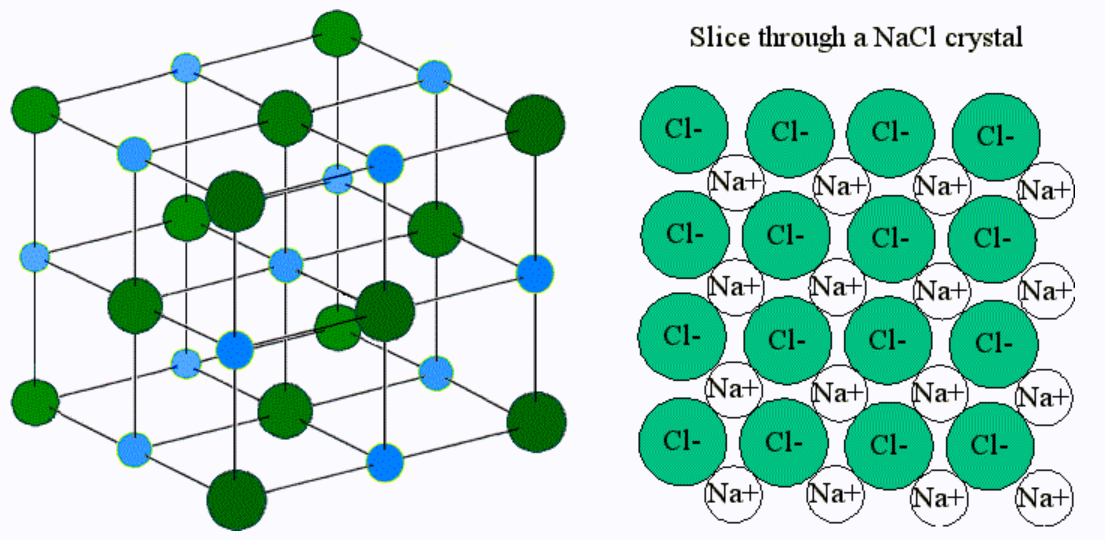
molecules
Ionic compounds do not form true ___________ because of the way in which the oppositely charged ions arrange themselves in the solid state as crystal lattices
iron(II), ferrous
Name the following ionic compound:
Fe2+ (2 names)
iron(III), ferric
Name the following ionic compound:
Fe3+ (2 names)
hydride
Name the following ionic compound:
H-
oxide
Name the following ionic compound:
O2-
nitride
Name the following ionic compound:
N3-
nitrite
Name the following ionic compound:
NO2-
nitrate
Name the following ionic compound:
NO3-
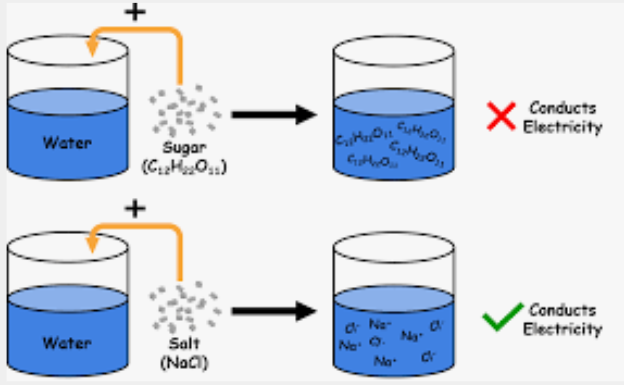
poor, lattice, crystalline, lattice, ion-dipole, solution, conduct
Solid ionic compounds tend to be ______ conductors of electricity because the charged particles are rigidly set in place by the ________ arrangement of the ____________ solid.
In aqueous solutions, however, the ________ arrangement is disrupted by the ____-_______ interactions between the ionic compounds and the water molecules.
The cations and anions are now free to move, and as a result, a _________ of ions is able to ________ electricity.
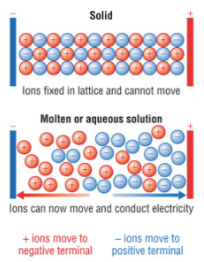
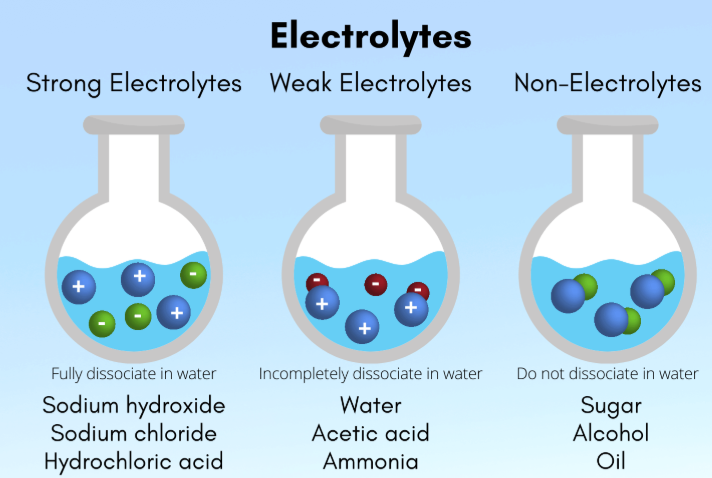
electrolytes

Solutes that enable solutions to carry currents
A substance that produces ions when dissolved in a polar solvent (like water), allowing the solution to conduct electricity through the movement of these ions (cations and anions)
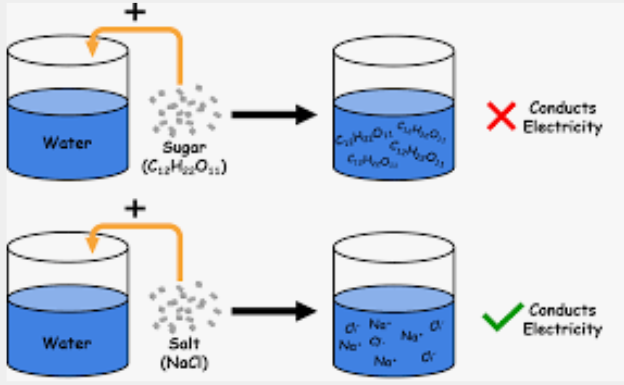
strong
Strong vs. Weak
A solute is considered a _______ electrolyte if it dissociates completely into its constituent ions
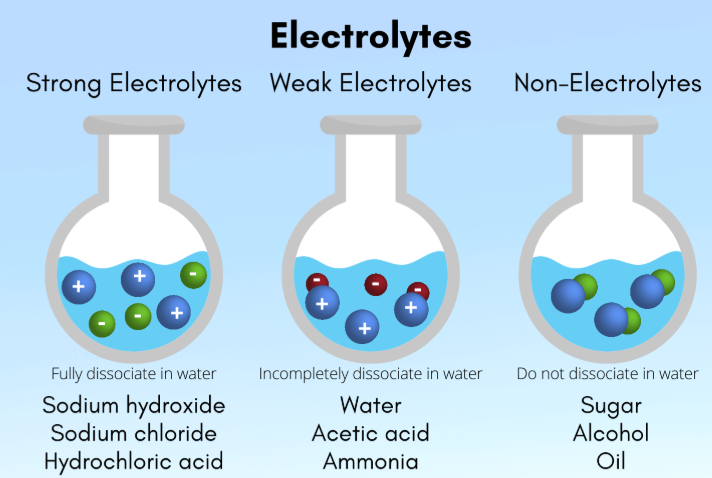
weak
Strong vs. Weak
A solute is considered a _______ electrolyte if it ionizes or hydrolyzes incompletely in aqueous solution, and only some of the solute is dissolved into its ionic constituents
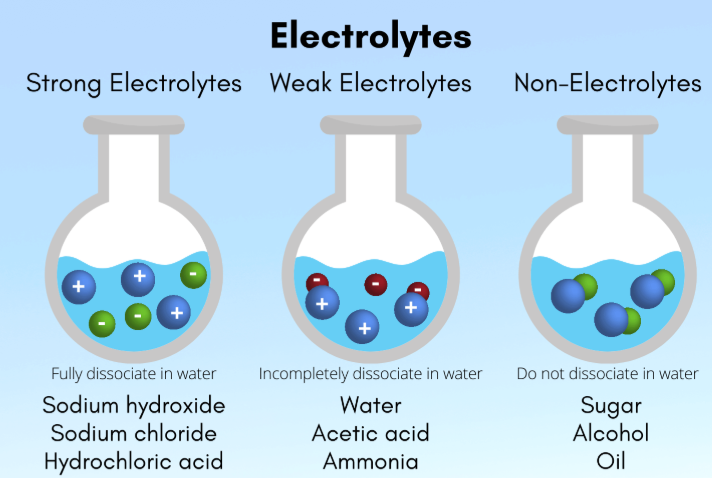
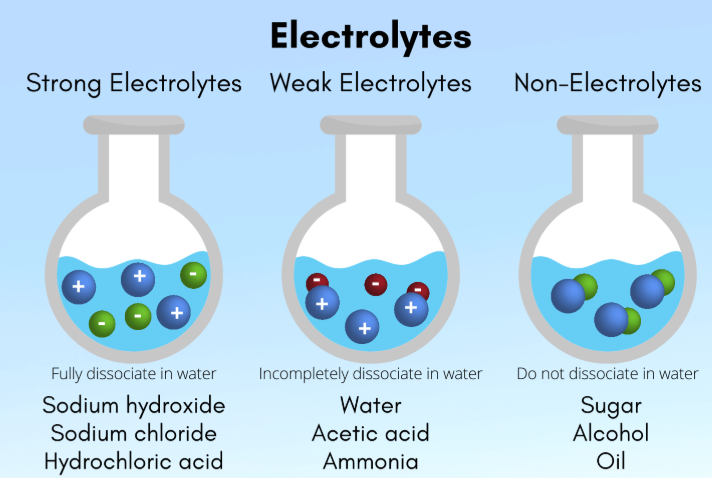
nonelectrolytes
Compounds that DO NOT ionize at all in water, retaining their molecular structure in solution
Includes many nonpolar gases and organic compounds