Lecture 2
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
where does spreading occur
at liquid-liquid interfaces
what is the extent of spreading determined by
spreading coeffcient
what must the spreading coefficent (S) be equal to for spreading to occur
equal to or greater than 0
what is cohesion
the forces in a single liquid
what is adhesion
forces between two immiscible liquids
what is the work of cohesion (W c)
the work required to overcome forces within one liquid
when does spreading only occur
if the work of adhesion is greater than the work of cohesion
spreading coefficent equation (s)
Wa - Wc
work of adhesion equation (Wa)
Wa = yA +yB - yA/B
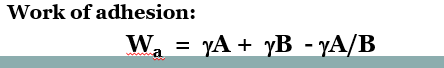
combining work of adhesion and spreading coefficient
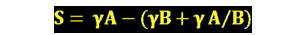
what is wetting
liquid - solid interfaces
what will a drop of liquid adsorbed on to a solid exhibit
a definite contact angle
the higher the contact angle
the poorer the wetting
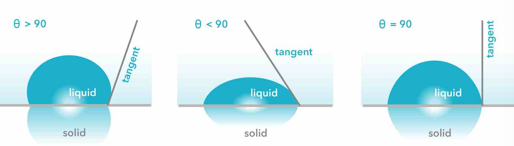
what is youngs equation used for
calculating the wetting of a solid
what does adsorbed mean
a liquid sitting on the surface of the solid
what is the equation to see if wetting occurs
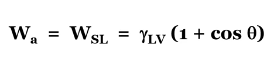
if the contact angle is 180 what is the wetting
0
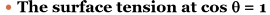
what is it called if surface tension at cos 0 = 1
critical surface tension
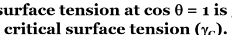
what is the work of adhesion (Wa)
work of adhesion refers to the newly created surface tension forces between two liquids
what is the inital and final spreading coefficient?
two immiscible liquids eventually become mutally saturated in one another, therefore there is an initial spreading coeffeicent (Sinital) and a final spreading coefficent (Sfinal) after mutual saturation
explain the difference in the type of systems work of adhesion refers to compared to the work of cohesion.
The difference between work of cohesion and work of adhesion is that the work of cohesion relates to a single liquid, the work of adhesion relates to two immiscible liquids.
what is the spreading coefficent
is the work of adhesion minus the work of cohesion
what is the pharmaceutical importance of wetting and spreading
Spreading is important in the formulation of creams.
Wetting is important in the dissolution of hydrophobic drugs.
explain the thermodynamic basis of micelle formation
Micelles form as systems want to achieve minimum free energy (G) and tend towards maximum entropy (S).
Before a surfactant is added a liquid will have minimum free energy and tend towards maximum entropy as it will have a defined structure due to the intermolecular bonding between the liquid molecules.
what is a micelle
A one tailed surfactant is dissolved in water.
what is a reverse micelle
A one tailed surfactant is dissolved in chloroform.