Acronyms
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
AAC
Actual Acquisition Cost
AARP
American Association of Retired Persons
ADE/ADR
Adverse Drug Experience/Adverse Drug Reaction
AMP
Average Manufacture Price
ANDA
Abbreviated New Drug Application
ATF
Alcohol, Tobacco, and Firearms agency
AWP
Average Wholesale Price
CAM
Complimentary Alternative Medicine
CDC
center for disease control
CDER
center for drug evaluation and research
CFR
Code of federal regulations
CMS
Centers for Medicaid and Medicare Services
CPSC
Consumer Product Safety Commission
CSOS
controlled substance ordering system
DEA
Drug Enforcement Agency
DHHS
Department of Human and Health Services
DI
Dietary Ingredient
DME
Durable Medical Equipment
DQSA
Drug Quality and Security Act
DRG
Diagnosis Related Groups
DS
Dietary Supplement
DSHEA
Dietary Supplement Health Education Act
DUR
Drug Utilization Review
EAC
Estimated Acquisition Cost
EHR
Electronic Health Records
EOB
Electronic Orange Book
FDA
Food and Drug Administration
FDCA
Food, Drug, and Cosmetic Act
FTC
federal trade commission
GMP
good manufacturing practice
GRAS
generally recognized as safe
GRASE
generally recognized as safe and effective
HCFA
health care financing administration
HHA
home health agency
HIPPA
Health Insurance Portability and Accountability Act
HMO
Health Maintenance Organization
HP/HPPUS
Homeopathic Pharmacopeia of the US
HSA
Health Savings Account
HSD
Home use sterile drug products
IND
investigational new drug development
JCAHO
Joint commission on accreditation of health care organizations
LVP
large volume parenteral
MAC
maximum allowable cost
NABP
national association of boards and pharmacy
NDA
new drug application
NDC

NDI
New Dietary Ingredient
NIH

Non DI
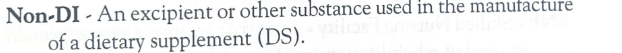
NPI

OBRA

OSHA
OTC
PBM

PFFS

PHS

PI

PPI
Patient Package Insert
PPO

PPPA
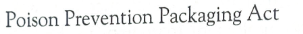
REMS
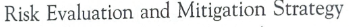
SDA

SNDA
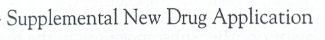
SNF

SNP

SP

TCAM
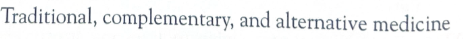
TRP

USAN

USP/NF

USP DI

VIPPS