6 common biological functional groups (study for quiz)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Hydroxyl group importance?
Polar; forms hydrogen bonds, Present in sugars and some amino acids.
Carbonyl group importance?
Polar, Present in sugars.
Carboxyl group importance?
Polar, Acidic, Present in fatty acids, and amino acids.
Amino group importance?
Polar, Basic, Present in amino acids.
Sulfhydryl group importance?
Slightly polar, forms disulfide covalent bonds, present in some amino acids.
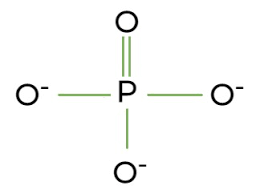
Phosphate group importance?
Polar, acidic, present in nucleotides and phospholipids.
Example of hydroxyl?
Ethanol.
Example of carbonyl group?
Acetone.
Example of carboxyl group?
Acetic acid.
Example of amino group?
Glycine.
Example of Sulfhydryl group?
Cysteine.
Example of Phosphate group?
Glycerol phosphate.
Chemical formula of Hydroxyl group?
-OH.
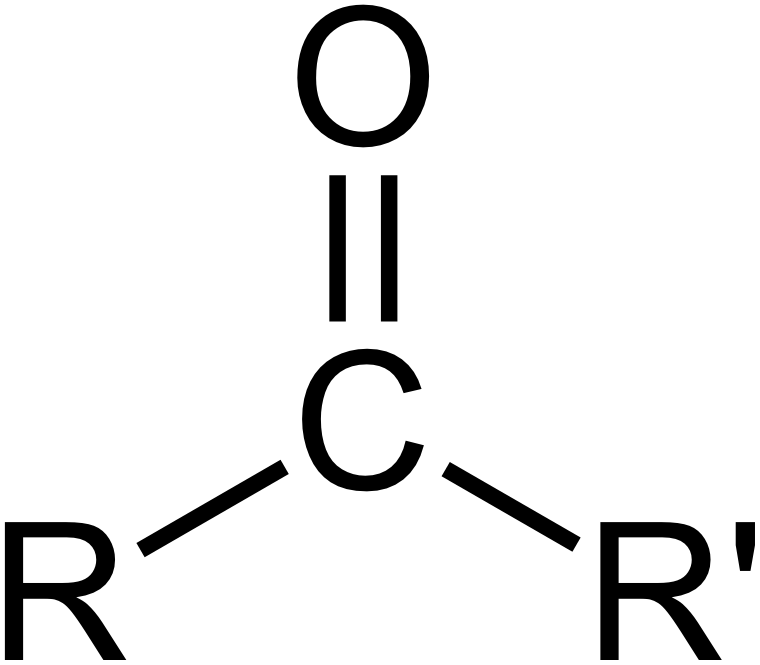
Chemical formula of Carbonyl group?
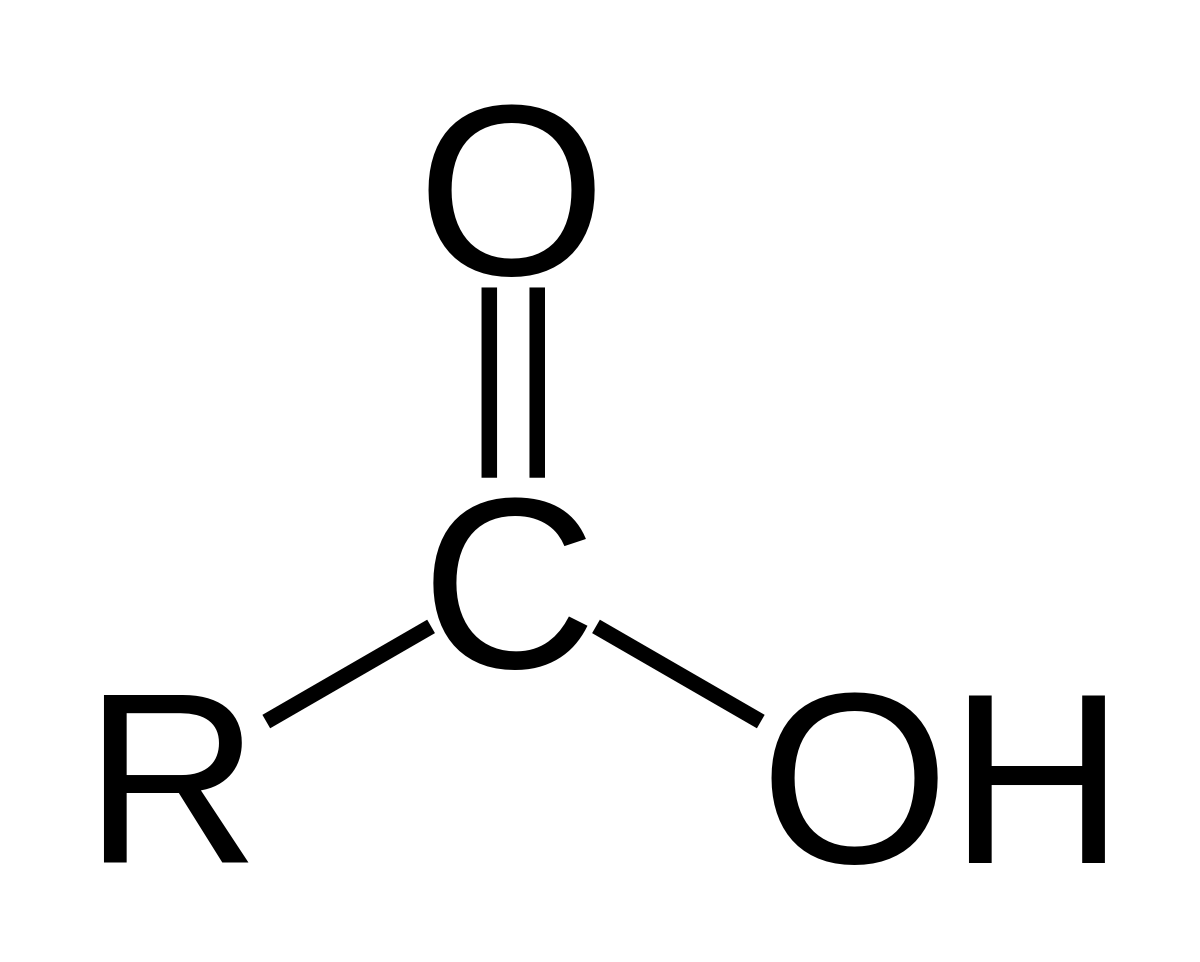
Chemical formula of Carboxyl group?
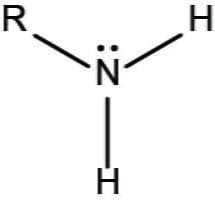
Chemical formula of Amino group?
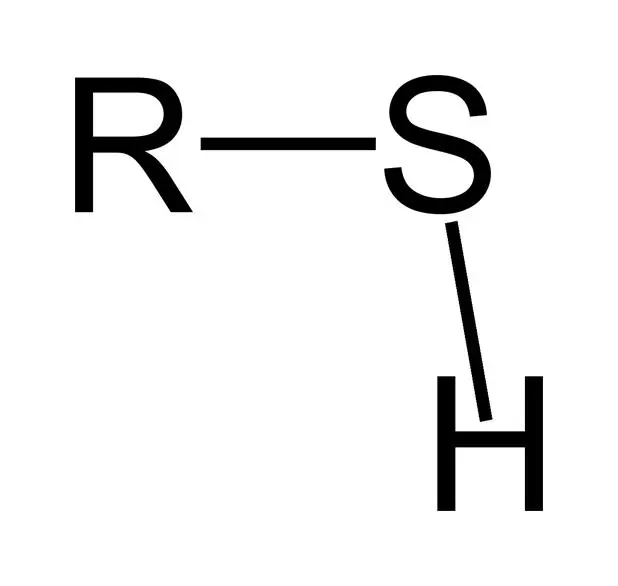
Chemical formula of Sulfhydryl group?
Chemical formula of Phosphate group?