Lab 8 (Sodium Borohydride Reduction)
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
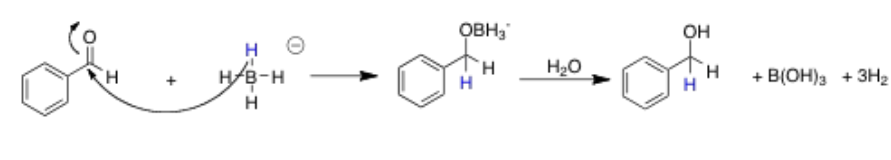
What is the mechanism of reduction via sodium borohydride
Proton from nucleophile (NaBH4) attacks the aldehyde group
Then the BH3 will attach to the O
This is then treated with water (or even better acid) to replace the BH3 with H to make it into an alcohol
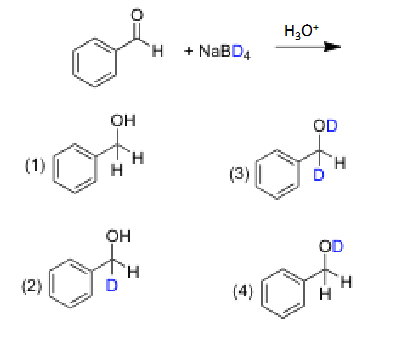
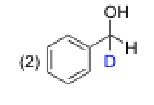
2, because the acid is what protonates the oxygen, the proton from NaBH4 only attacks the carbonyl carbon
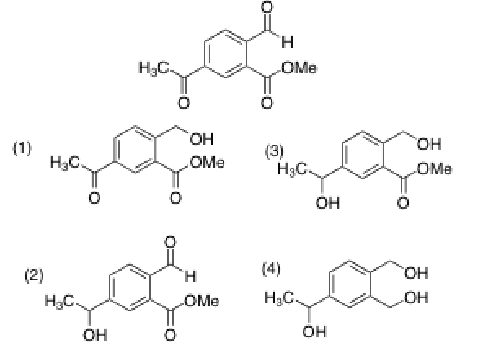
Suppose, you treated the following compound with a huge excess of sodium borohydride in methanol. What product do you expect to obtain?
3, because NaBH4 is not strong enough to reduce esters (need LiAlH4)
In principle, how many aldehyde molecules can one molecule of NaBH4 reduce?
4, one for each proton

Why it is better to use 1 M aqueous NaOH solution as a solvent for NaBH4 reduction rather than pure water?
4, because NaBH4 typically reacts really fast so the NaOH slows this down