Immunology - Lecture 15 - Development of B lymphocytes and B cell tolerance
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Where does lymphocyte production occur after birth, and what is the goal of lymphopoiesis?
Central Lymphoid Tissues: Bone marrow produces B cells, and thymus produces T cells (both originate in the bone marrow).
Lymphopoiesis Goal: Develops diverse B and T cell receptors for adaptive immune responses.
Development Stages: High lymphocyte production in fetal/juvenile stages to populate peripheral tissues (e.g., lymph nodes, spleen).
Age Factor: Lymphocyte production slows with age.
Why is testing the antigen receptors on B and T cells critical, and what is positive selection?
Purpose of Testing Receptors: Ensures B and T cells recognize pathogens but not self-antigens, preventing autoimmunity.
Selection Process: Immature lymphocytes undergo selection to confirm specificity for foreign antigens.
Positive Selection: Weak interaction with self-antigens grants survival signals, allowing cells to join the mature repertoire.
Importance: Critical for developing functional alpha-beta T cells.
What role does negative selection play in lymphocyte development, and what happens to developing lymphocytes in the absence of receptor signals?
Negative Selection: Eliminates strongly self-reactive lymphocytes to prevent autoimmunity.
Goal: Ensures only non-self-reactive cells survive.
Default Fate: Without receptor signaling, developing lymphocytes undergo apoptosis, supporting self-tolerance.
What are the main stages of B lymphocyte development and the purpose of gene rearrangement in this process?
B lymphocyte development progresses in stages, each defined by assembling and expressing functional antigen receptor genes.
Each stage monitors gene rearrangement success.
Successful rearrangement produces a protein chain, signaling progression to the next phase.
Developing B cells have multiple chances for rearrangement, enhancing the likelihood of functional receptor formation.
Checkpoints ensure that each B cell expresses only one receptor specificity.
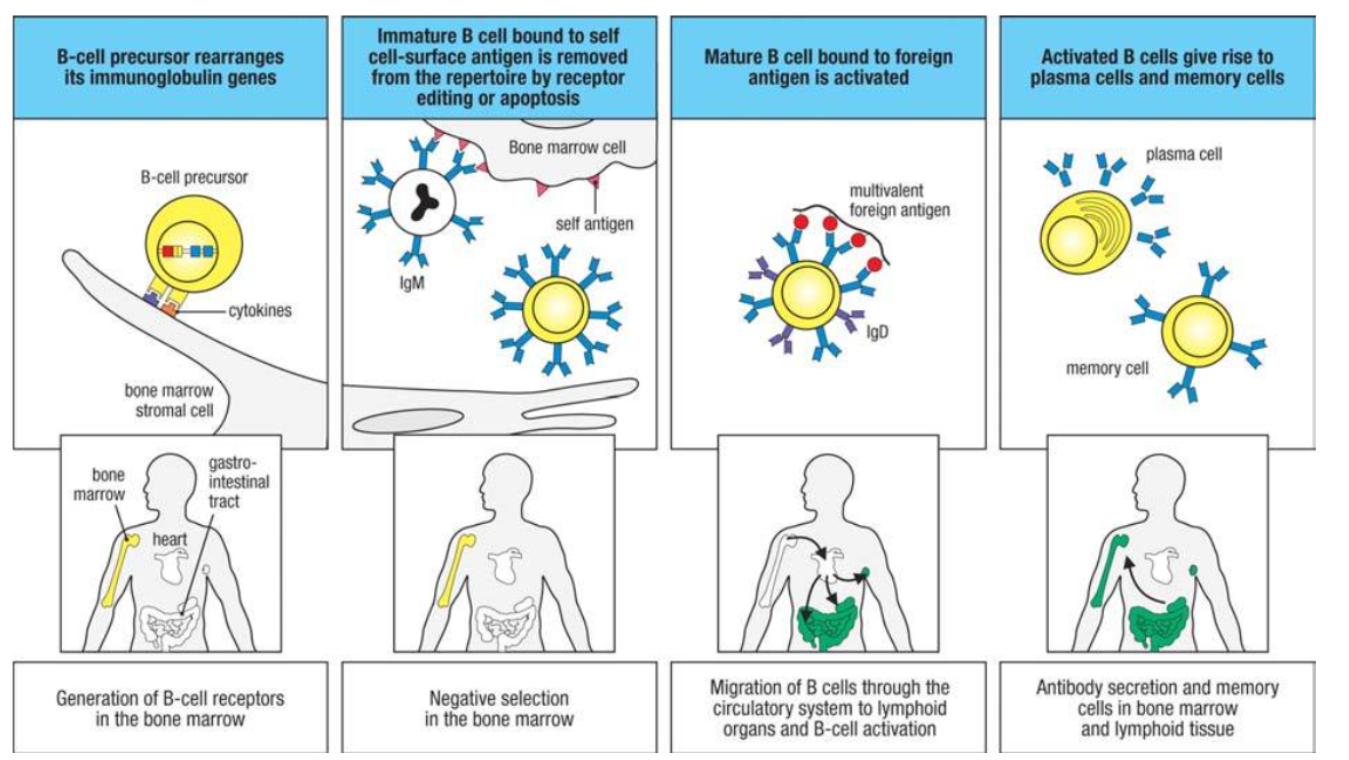
What are the main stages of B cell development and activation as shown in Figure 8.2?
B cells develop in the bone marrow, then migrate to peripheral lymphoid organs for activation.
Phases of Development:
Phase 1: Progenitor B cells rearrange Ig genes in the bone marrow, producing immature B cells with surface IgM.
Phase 2: Immature B cells interact with antigens; self-reactive cells undergo receptor editing or apoptosis to ensure self-tolerance.
Phase 3: Surviving B cells mature in the periphery, co-expressing IgD and IgM, and gain the ability to respond to foreign antigens.
Activation: Mature B cells, upon activation, proliferate and differentiate into antibody-secreting plasma cells and memory cells.
How does the bone marrow microenvironment support B cell development?
The bone marrow microenvironment supports lymphocyte development from HSCs and B cell differentiation.
Role of Stromal Cells:
Form adhesive contacts with developing lymphocytes.
Provide cytokines and chemokines to regulate differentiation and proliferation.
These signals activate genes essential for B cell development.
What role do bone marrow stromal cells play in early B cell development?
Bone Marrow Stromal Cells: Essential for B cell development from early progenitors to immature B cells.
Key Interactions:
FLT3 Signaling: FLT3 on progenitors binds FLT3 ligand on stromal cells, aiding in differentiation to common lymphoid progenitors.
CXCL12 Retention: Retains stem cells and progenitors near stromal cells.
IL-7 Support: IL-7 from stromal cells binds to IL-7 receptors, crucial for B cell development.
Adhesion & Proliferation:
Integrin VLA-4 on progenitors binds VCAM-1 on stromal cells, aiding adhesion.
SCF/Kit Activation: SCF on stromal cells activates Kit on progenitors, promoting proliferation.
How do hematopoietic stem cells (HSCs) progress toward lymphoid and myeloid lineages?
HSC Differentiation to LMPPs: LMPPs can produce lymphoid and myeloid cells but lack self-renewal.
Key Features & Pathways:
FLT3 Signaling: LMPPs express FLT3, binding FLT3 ligand on stromal cells.
Transcription Factors: Essential for various hematopoietic lineages.
Major Progenitor Subsets:
Early Innate Lymphoid Progenitor: Produces ILCs (ILC1, NK cells, ILC2, ILC3).
Common Lymphoid Progenitor (CLP): High IL-7 receptor expression; can become B cells, T cells, NK cells.
Early Thymic Progenitor (ETP): Expresses Notch protein, crucial for T cell lineage.
CLP Heterogeneity: CLP includes subsets that can specialize into B cells, T cells, or NK cells.
What is the role of IL-7 in the development of lymphocyte progenitors?
IL-7 Source: Secreted by bone marrow stromal cells.
Role: Essential for growth and survival of innate lymphoid cells and most developing T cells.
In Mice: Supports B cell development as well (may vary in humans).
IL-7 Receptor Expression: Found on lymphocyte progenitors starting from the LMPP stage.
How is commitment to the B cell lineage regulated in common lymphoid progenitors?
E2A Activation: Initiates B cell development by inducing FOXO1 expression in common lymphoid progenitors.
FOXO1 & E2A: Together promote early B cell factor (EBF) expression.
EBF Role: Amplifies B lineage gene expression by upregulating PAX5.
Transitional Phase: This transcriptional network transitions common lymphoid progenitors to pro-B cells.
Pro-B Cell Support: IL-7 provides survival and proliferation signals.
Maturation Path: B lineage cells mature within the bone marrow, then move to peripheral lymphoid organs like the spleen for final maturation.
What are the stages of B cell development, and which transcription factors regulate each step?
B cell development progresses through distinct stages, each regulated by specific transcription factors:
Early Pro-B Cell Stage: Heavy chain D-to-J joining begins. Regulated by transcription factors E2A and EBF, which activate RAG-1 and RAG-2 for initiating V(D)J recombination.
Late Pro-B Cell Stage: V-to-DJ joining occurs. PAX5 is activated by E2A and EBF, committing cells to the B cell lineage by inducing expression of CD19, Igα, and BLNK.
Large Pre-B Cell Stage: Expresses the pre-B cell receptor with the successfully rearranged heavy chain paired with surrogate light chains, which signals for cell proliferation.
Small Pre-B Cell Stage: RAG proteins are re-expressed, allowing light chain gene rearrangement, while the surrogate chain expression ceases.
Immature B Cell Stage: A complete IgM molecule is expressed on the cell surface, signaling readiness for antigen interaction.
Mature B Cell Stage: Achieved after migrating to the spleen, where cells express both IgM and IgD, completing their differentiation.
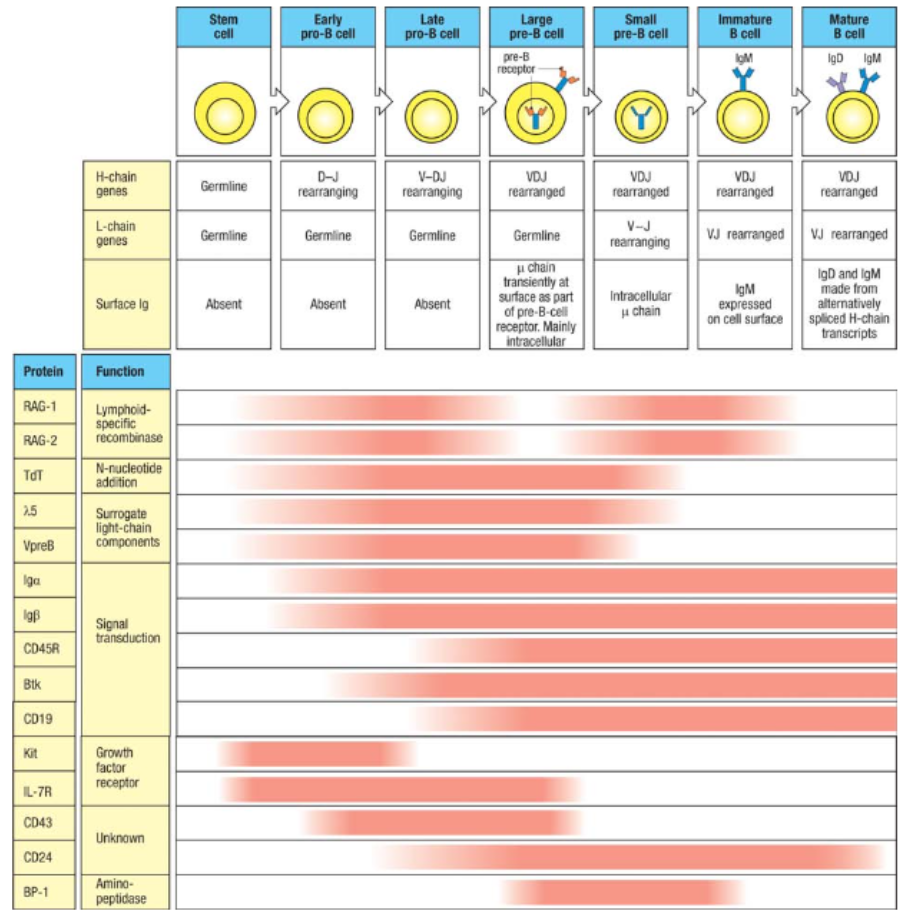
What are the key stages and markers in B cell development as shown in Figure 8.4?
B cell development progresses through multiple stages in the bone marrow:
Stem Cell: Ig genes in germline configuration.
Early Pro-B Cell: Initiates heavy chain D-to-J rearrangement; expresses early markers CD19, CD45R, CD43, Kit, and IL-7 receptor.
Late Pro-B Cell: V-to-DJ rearrangement; CD24 expression starts.
Large Pre-B Cell: Expresses pre-B cell receptor with surrogate light chains; proliferates and signals through Igα, Igβ, and Btk.
Small Pre-B Cell: Ceases surrogate chain expression, reexpresses RAG proteins, begins light chain rearrangement.
Immature B Cell: Expresses complete IgM on surface, signaling via Igα and Igβ.
Mature B Cell: Completes development in the spleen, co-expresses IgM and IgD through mRNA splicing.
Key markers evolve with each stage, facilitating the progression and survival of the developing B cell.
How does the V(D)J recombinase system function differently between B and T cells, and what is unique about D-to-J joining in B cells?
Shared V(D)J Recombinase System: Operates in both B and T cells but with lineage-specific rearrangements.
B Cells:
T cell receptor genes do not rearrange.
First, D-to-J joining occurs in the immunoglobulin heavy chain locus on both alleles.
This D-to-J joining progresses the cell to the pro-B cell stage.
T Cells:
Immunoglobulin genes do not rearrange.
Human Specificity:
Most D-to-J joins are functional in humans, as D gene segments translate across reading frames without stop codons.
Low-level transcription near gene segments helps regulate lineage-specific rearrangement.
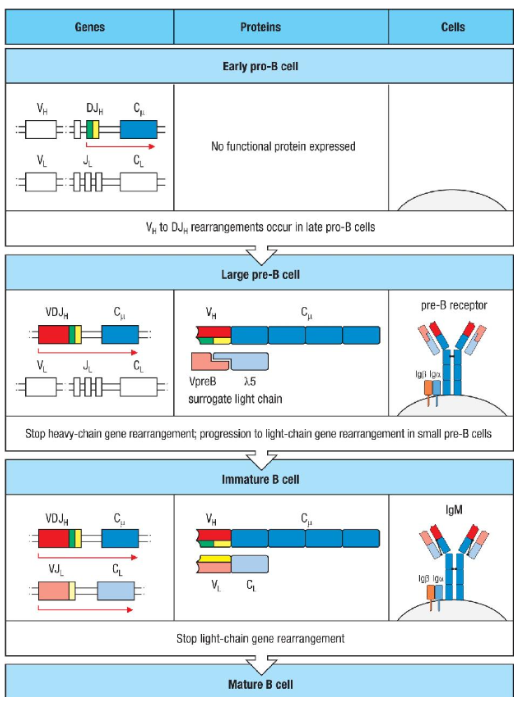
What are the stages of immunoglobulin gene rearrangement in B cell development, and what triggers each transition?
B cell development progresses through specific stages of gene rearrangement:
Early pro-B cell: Initiates heavy chain rearrangement with D-to-J joining. No functional protein is produced yet.
Late pro-B cell: V-to-DJ rearrangement occurs first on one chromosome. If unsuccessful, it happens on the second chromosome. A functional heavy chain leads to mu chain production.
Large pre-B cell: The mu chain combines with surrogate light chains (lambda 5 and VpreB) to form the pre-B cell receptor, which pairs with Igα and Igβ to signal the cell to halt heavy chain rearrangement and begin proliferation.
Small pre-B cell: Light chain rearrangement starts with V-to-J joining. A successful rearrangement results in a complete IgM molecule.
Immature B cell: IgM, now paired with Igα and Igβ on the cell surface, signals to stop further light chain rearrangement, leading to maturation.
Key Points: Failure at any step in producing functional heavy or light chains leads to cell death.
How does the late pro-B cell stage ensure the successful production of a complete immunoglobulin heavy chain?
In the late pro-B cell stage, V-to-DJ rearrangement occurs initially on one chromosome. If successful, this produces an intact mu heavy chain, allowing the cell to progress to the pre-B cell stage and receive survival signals through the pre-B cell receptor.
If the first V-to-DJ rearrangement is non-productive (occurring out of frame), the cell attempts rearrangement on the other chromosome.
Due to the triplet nature of genetic encoding, there’s a 1 in 3 chance of an in-frame junction with each rearrangement attempt.
Survival Rate: Approximately 55% of pro-B cells succeed in producing a pre-B cell, with at least 45% being lost due to failure in producing a functional mu chain.
Some pseudogenes within the gene segment repertoire can also interfere, reducing the chance of a functional protein.
Sequential Rearrangements: Pro-B cells can undergo multiple rearrangements on both chromosomes to maximize the chance of producing a functional heavy chain before elimination.
How does terminal deoxynucleotidyl transferase (TdT) contribute to the diversity of the B cell antigen receptor repertoire?
TdT enhances B cell receptor diversity by adding non-templated nucleotides (N-nucleotides) at the joints between rearranged gene segments during heavy chain gene rearrangement.
Expression: TdT is expressed in pro-B cells during heavy chain rearrangement, but its expression declines in pre-B cells during light chain rearrangement.
Nucleotide Addition: This enzyme contributes N-nucleotides to the V-D and D-J joints of nearly all heavy chains in adult humans, but only about 25% of human light chain joints contain N-nucleotides.
What is the role of the pre-B cell receptor (pre-BCR) in early B cell development?
The pre-B cell receptor (pre-BCR) serves as a checkpoint in early B cell development, verifying the successful production of a functional heavy chain via VDJ recombination.
Checkpoint Function: The pre-BCR tests for a productive heavy chain, a process prone to errors and nonfunctional rearrangements.
Surrogate Light Chains: At this stage, light chains have not yet rearranged. Pro-B cells express two surrogate proteins, lambda 5 and VpreB, which resemble light chains and pair with the mu heavy chain to form the pre-BCR.
Regulation: The expression of these surrogate chains is regulated by transcription factors E2A and EBF.
Outcome: Successful assembly of the pre-BCR signals the transition from the pro-B cell stage to the pre-B cell stage in B cell development.
How does the pre-B cell receptor (pre-BCR) signal successful heavy chain rearrangement, and why is it important?
The pre-BCR signals successful heavy chain rearrangement independently of external antigens. This signaling is crucial for B cell development and is achieved through spontaneous dimerization of pre-BCRs, facilitated by unique regions of lambda 5 and VpreB.
Signaling Pathway: The dimerization of pre-BCRs leads to cross-linking that activates intracellular pathways via the signaling molecules Ig alpha and Ig beta.
Key Proteins: BLNK (a scaffold protein) and Btk are critical for transducing these signals, enabling the transition from pro-B cell to pre-B cell.
Significance: This signaling verifies functional heavy chain production, a necessary step for B cell maturation, and helps establish a functional and diverse antibody repertoire for the immune system.
What is the purpose of Pre-B cell receptor (Pre-BCR) signaling in B cell development, and what outcomes does it achieve?
The purpose of Pre-BCR signaling is to act as a checkpoint that ensures a functional heavy chain has been produced.
Mechanism: Clustering of Pre-BCRs on the cell surface generates the necessary signaling.
Outcomes:
Halts further heavy chain gene rearrangement, enforcing allelic exclusion so only one heavy chain allele is expressed per B cell.
Promotes cell proliferation, allowing successful B cells to expand in number before proceeding to light chain rearrangement.
This checkpoint is essential for ensuring each B cell expresses a single, unique antibody specificity.
What is allelic exclusion, and why is it important in B cell development?
Allelic exclusion is a process in which only one allele of a gene is expressed in diploid cells. This ensures that each B cell produces immunoglobulins with a single antigen specificity.
Importance: Prevents a single B cell from expressing receptors with different antigen specificities, which could dilute the immune response and reduce effectiveness.
Occurrence: Allelic exclusion is enforced at both the heavy chain and light chain loci during B cell development, ensuring that each B cell has a unique antibody specificity.
How does Pre-BCR signaling enforce heavy chain allelic exclusion?
Pre-BCR signaling enforces allelic exclusion of the heavy chain through three main mechanisms:
Reduction of V(D)J Recombinase Activity
Action: Pre-BCR signaling downregulates Rag1 and Rag2 expression, reducing recombination activity.
Degradation of RAG-2 Protein
Process: Signaling triggers phosphorylation of RAG-2 during the S phase of the cell cycle, leading to degradation of RAG-2, further decreasing recombinase activity.
Reduced Accessibility of Heavy Chain Locus
Effect: Alters chromatin structure to make the heavy chain locus less accessible to recombination machinery, thereby preventing additional rearrangements.
This process ensures that each B cell expresses only one functional heavy chain, supporting allelic exclusion and preserving single antigen specificity.
What evidence supports allelic exclusion in B cells?
Allotypes provide evidence for allelic exclusion in B cells.
Definition: Allotypes are genetic polymorphisms in the immunoglobulin constant regions, resulting in different variants.
Experiment: In heterozygous animals (with both allotype a and b alleles), individual B cells express either allotype a or allotype b, but not both.
Conclusion: This finding supports that each B cell expresses immunoglobulin from only one heavy chain allele, ensuring single antigen specificity.
How does Pre-BCR signaling promote B cell proliferation?
Pre-BCR signaling stimulates the expansion of B cells with a successful heavy chain rearrangement by:
Transitioning to the large pre-B cell stage where proliferation is promoted.
IL-7 Response: Cells respond to IL-7, undergoing multiple rounds of cell division.
Expansion Rate: Results in a population increase of 30-60 fold.
This proliferation stage ensures that cells with a functional heavy chain multiply before further development.
When and how does RAG expression reinitiate for light chain rearrangement in B cells?
RAG expression is reactivated after proliferation, as cells transition to the small pre-B cell stage.
Selective Rearrangement: RAG proteins are re-expressed to rearrange light chain loci, allowing for light chain gene rearrangement.
Heavy Chain Locus: Remains inactive, ensuring no further rearrangement occurs on the heavy chain.
This process enables the formation of a complete B cell receptor with a functional light chain while maintaining heavy chain allelic exclusion.
What are the implications if Pre-BCR signaling is absent in B cell development?
Absence of Pre-BCR signaling has critical consequences:
Failure of Allelic Exclusion: Without Pre-BCR signals, heavy chain allelic exclusion fails, potentially leading to B cells that express receptors from both heavy chain alleles.
Developmental Block: B cell development is arrested at the pro-B cell stage, resulting in immunodeficiency due to the lack of mature B cells.
This checkpoint is essential for ensuring proper B cell maturation and immune functionality.
What are the key functions of Pre-BCR signaling and allelic exclusion in B cell development?
Pre-BCR signaling is essential for:
Enforcing allelic exclusion at the heavy chain locus, ensuring each B cell produces a unique antigen receptor.
Halting further heavy chain gene rearrangement once a productive rearrangement is achieved.
Stimulating proliferation of pre-B cells, expanding the pool with functional heavy chains in response to IL-7.
Mechanisms to Remember:
Downregulation and degradation of RAG proteins via Pre-BCR signaling.
Reduction of heavy chain locus accessibility to recombinase machinery.
Proliferation driven by IL-7 following Pre-BCR signaling.
These mechanisms ensure that B cells have specific and effective antigen receptors, maintaining a focused immune response.
What occurs during the light chain rearrangement in pre-B cells, and how does this contribute to antigen specificity?
After several rounds of division, large pre-B cells become small resting pre-B cells and re-express RAG proteins, allowing light chain rearrangement.
Light Chain Rearrangement: Occurs by V-to-J joining without D segments. If a rearrangement is nonfunctional, additional V and J segments on the same allele can be rearranged.
Allelic Exclusion: Light chain rearrangement typically occurs on one allele at a time, ensuring that each B cell produces a unique receptor. This process is regulated by an as-yet-unknown mechanism.
Antigen Specificity: Each pre-B cell can produce progeny with distinct light chains, enhancing antigen receptor diversity. The presence of two light chain loci further increases the likelihood of producing a functional light chain.
Outcome: Cells that successfully generate a functional light chain express IgM on the cell surface, classifying them as immature B cells ready for antigen testing.
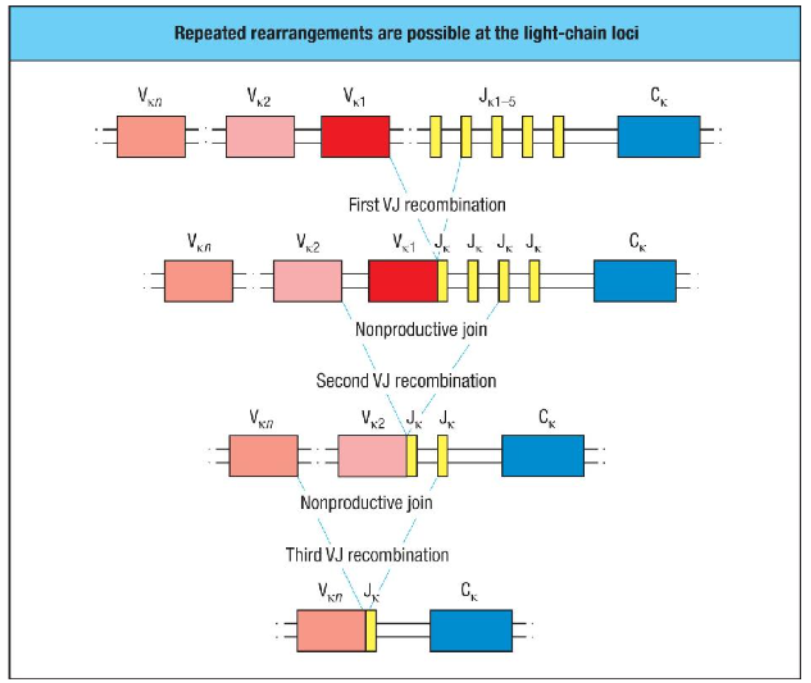
How can nonproductive light chain gene rearrangements be rescued during B cell development?
Nonproductive light chain rearrangements can undergo further rearrangements for rescue:
Process: If an initial V-to-J rearrangement is nonproductive, a new V segment can combine with an unused J segment to bypass the faulty join.
Opportunity for Rearrangement: This process can repeat up to five times per chromosome in humans and mice, offering multiple chances for generating a productive light chain.
Outcome: If all kappa chain rearrangements are unsuccessful, the lambda light chain loci may be rearranged as a final attempt to produce a functional light chain.
Importance: This mechanism enhances the likelihood that pre-B cells will produce a functional light chain, enabling them to progress in B cell development.
What process ensures that immature B cells are tolerant of self-antigens before they leave the bone marrow?
Immature B cells, after pairing a rearranged light chain with a mu heavy chain, express IgM on their surface and are tested for autoreactivity:
Stage: This occurs at the immature B cell stage, where the antigen receptor is tested against self-antigens.
Outcome: Autoreactive B cells are eliminated at this stage, ensuring self-tolerance and reducing the risk of autoimmunity.
Type of Tolerance: This elimination process establishes central tolerance, as it occurs in the central lymphoid organ—the bone marrow.
Further Maturation: B cells that pass this test are not fully mature and will complete additional maturation in peripheral lymphoid organs.
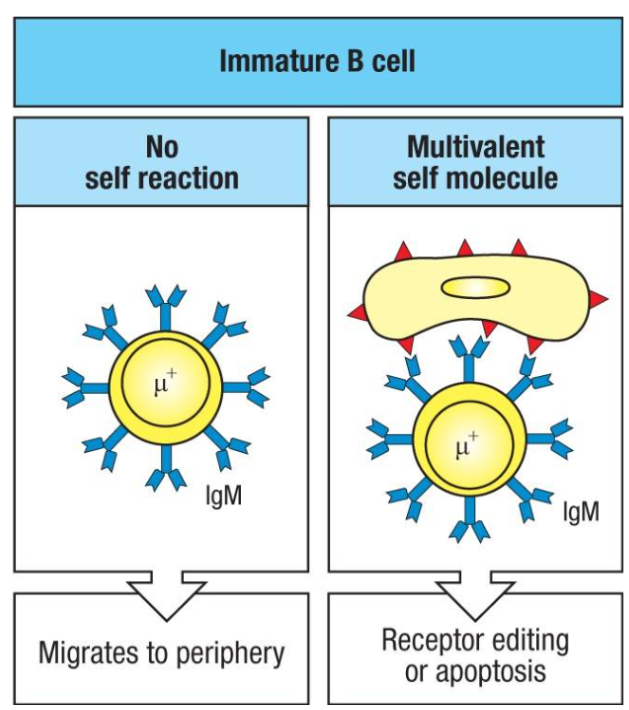
What happens to immature B cells in the bone marrow if they bind to self molecules?
Immature B cells can have two possible fates in response to self molecules:
No Self-Reaction (Left): B cells that do not encounter self-antigens continue to mature and migrate to peripheral lymphoid tissues, where they express both IgM and IgD and may become mature recirculating B cells.
Reaction to Multivalent Self Molecules (Right): If B cells bind multivalent self-antigens, they either:
Undergo Receptor Editing: Modify their antigen receptor to remove self-reactivity.
Undergo Apoptosis: If editing fails, they undergo programmed cell death (clonal deletion) to prevent autoimmunity.
What happens to immature B cells that show no strong reactivity to self antigens, and what occurs if they encounter a strongly cross-linking antigen?
Non-reactive Immature B Cells: Mature, leave the bone marrow, and enter circulation if they do not strongly react to self-antigens.
Strong Self-Reactivity: Immature B cells that encounter strongly cross-linking self-antigens in the bone marrow experience developmental arrest.
Receptor Editing: Autoreactive B cells undergo further gene rearrangement to replace the self-reactive receptor with a non-autoreactive one.
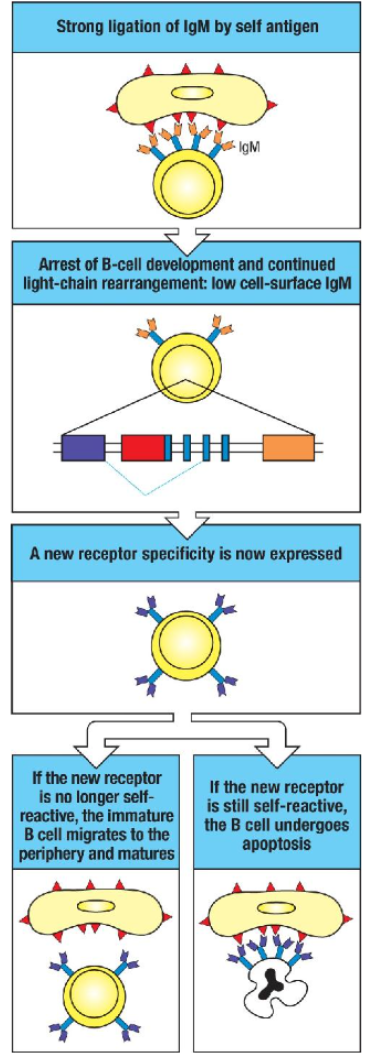
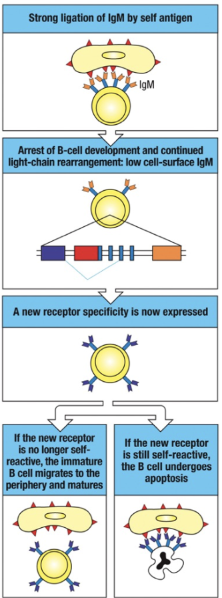
How does receptor editing function as a mechanism of central tolerance for B cells in the bone marrow?
Central Tolerance Mechanism: Receptor editing is key for ensuring B cell tolerance.
Self-reactive IgM: Initial self-reactive IgM receptors allow further light chain rearrangement due to continued RAG protein expression.
Non-reactive Outcome: If a new receptor is non-reactive, gene rearrangement stops, RAG proteins disappear, and development proceeds.
Ongoing Autoreactivity: If still autoreactive, IgM cross-linking maintains RAG expression, permitting further light chain rearrangement to replace the self-reactive chain.
Failed Editing: If autoreactivity persists after multiple attempts, the B cell undergoes apoptosis (clonal deletion).
Disease Link: Defective receptor editing can result in autoimmune diseases.
How does receptor editing prevent self-reactivity in developing B cells?
Receptor Editing Trigger: Strong self-antigen cross-linking of a B cell receptor initiates receptor editing.
Process Details: Surface IgM expression decreases, and RAG protein production continues, allowing further light chain rearrangement.
Outcome of Editing:
Non-reactive Receptor: If the new receptor is not self-reactive, the B cell resumes normal development.
Persistent Self-reactivity: If still self-reactive, the B cell may attempt additional rearrangements.
Final Fate: If self-reactivity remains after attempts, the cell undergoes apoptosis (clonal deletion), removing the autoreactive B cell.
What is peripheral tolerance, and why is it important in B cell development?
Peripheral Tolerance: Eliminates self-reactive B cells that encounter self-antigens in peripheral tissues.
Distinction from Central Tolerance: Central tolerance in the bone marrow removes many self-reactive B cells, but some antigens (e.g., thyroglobulin) are only encountered outside the bone marrow.
Purpose: Prevents autoimmune responses by eliminating self-reactive B cells after they leave the bone marrow, maintaining immune self-tolerance.
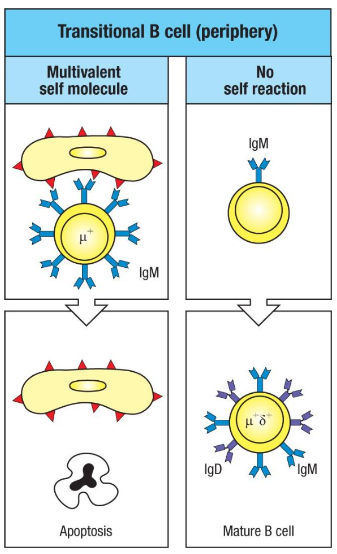
What happens to transitional B cells that recognize self-antigens in the periphery?
Transitional B Cells: Newly emigrated from the bone marrow and still tested for self-reactivity in the periphery, especially in the spleen.
Tolerance Testing: Encounter with multivalent self-antigens triggers a strong IgM signal, leading to apoptosis if self-reactive.
Maturation: Non-self-reactive cells upregulate IgD and mature fully, residing in B cell follicles in the spleen.
Purpose: Eliminates self-reactive B cells, reinforcing peripheral tolerance.

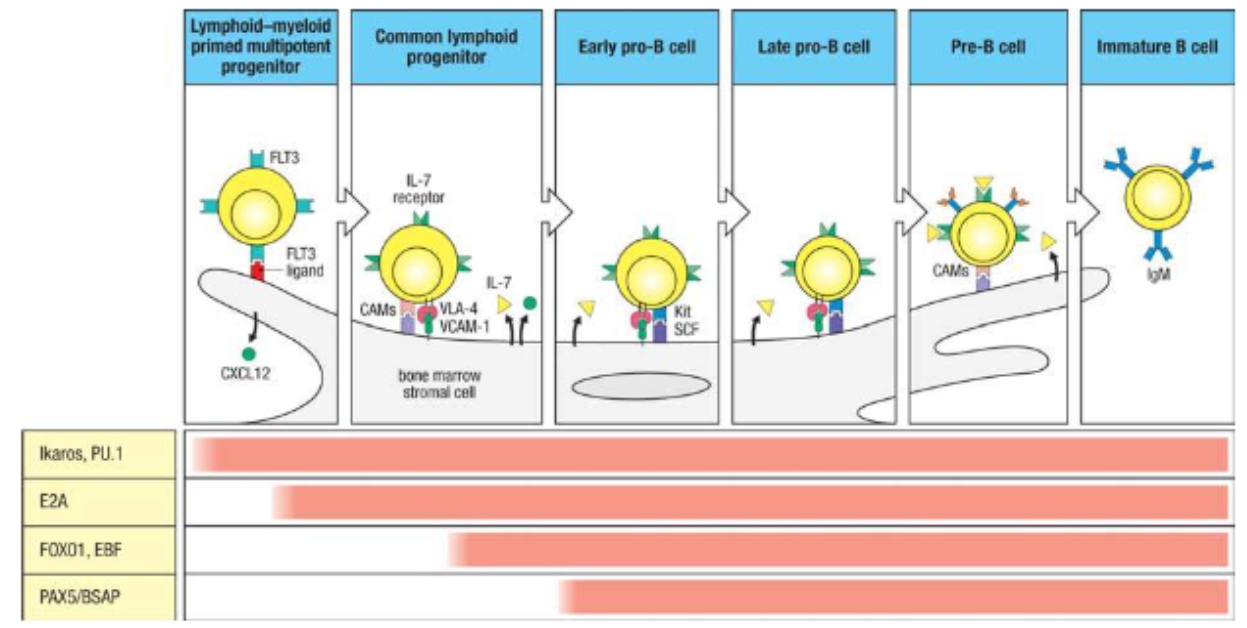
What is the first step of B cell development?
Starting Stage: Lymphoid-Primed Multipotent Progenitor (LMPP).
Key Transcription Factors: Ikaros, PU.1, and Kit.
Commitment: Signals commitment to lymphoid lineage, but not specifically to B cells yet.
Differentiation Potential: LMPP can develop into various lymphoid cells, including B cells.
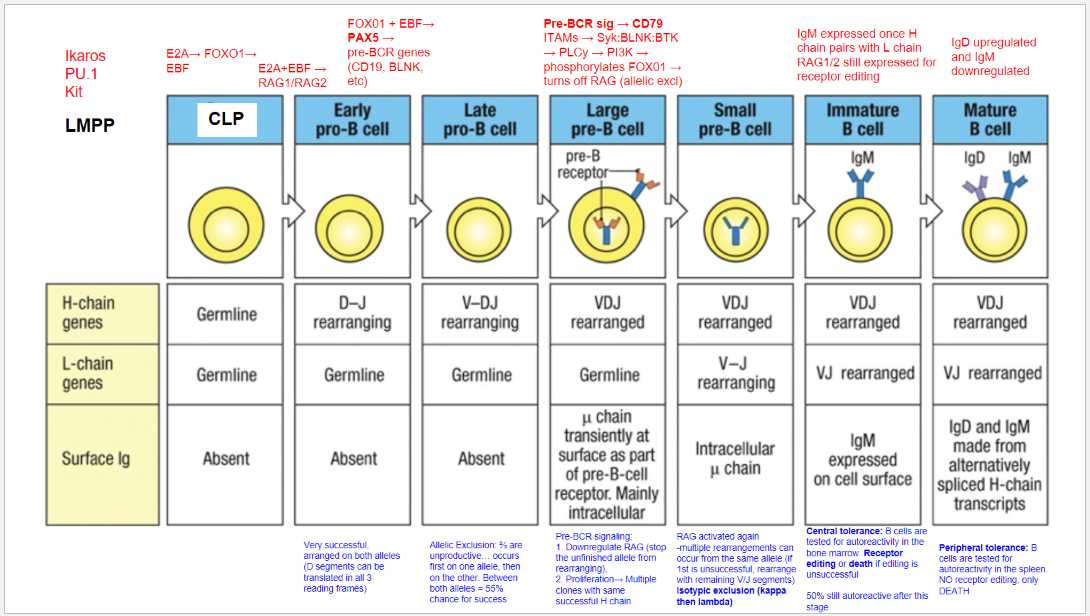
What is the second step of B cell development?
Stage: Common Lymphoid Progenitor (CLP).
Key Transcription Factors: E2A, FOXO1, and EBF.
Commitment: Begins commitment to B cell lineage.
Role of EBF: Critical for early B cell differentiation.
Role of E2A: Initiates expression of genes, including RAG1 and RAG2, necessary for immunoglobulin gene rearrangement.
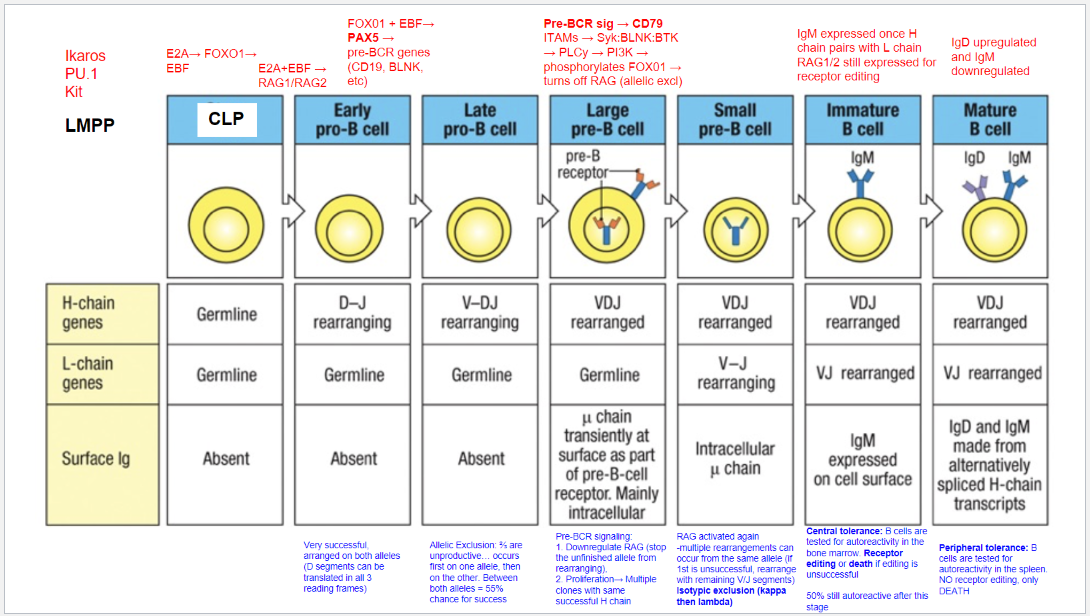
What is the third step of B cell development, and what occurs at the Early Pro-B Cell stage?
Stage: Early Pro-B Cell.
Key Transcription Factors: E2A and EBF.
Activated Enzymes: RAG1 and RAG2 initiate recombination.
Heavy Chain Rearrangement: D and J segments rearrange in the heavy chain gene.
Translation Flexibility: D segments can be translated in all reading frames, increasing successful rearrangement chances.
Light Chain Status: Remains in germline configuration; L-chain rearrangement hasn’t started.
Surface Immunoglobulin: Absent at this stage.
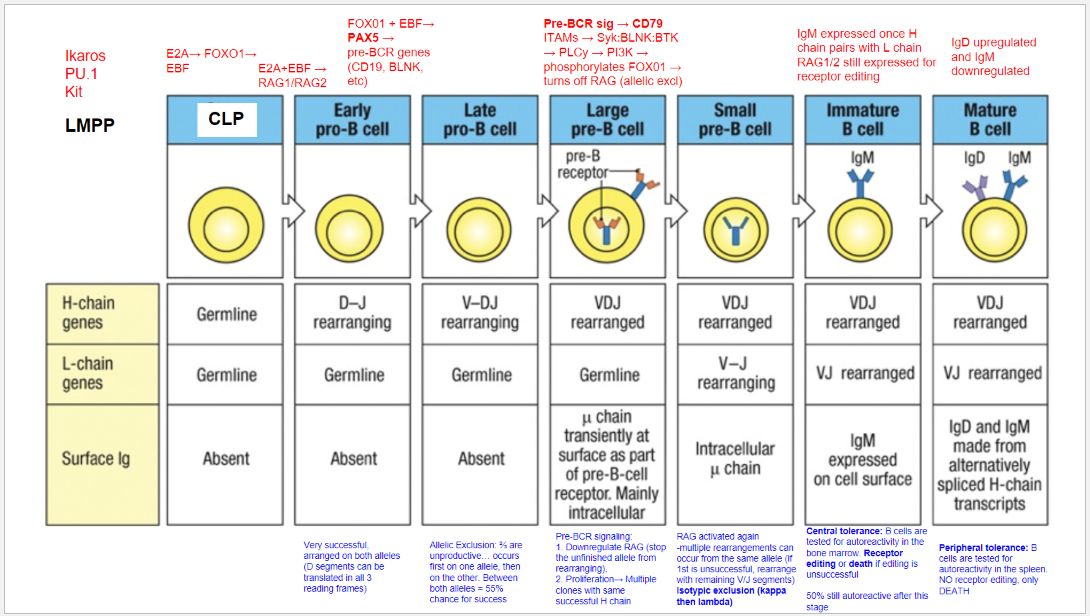
What occurs at the fourth step, the Late Pro-B Cell stage, in B cell development?
Stage: Late Pro-B Cell.
Key Transcription Factors: FOXO1 and EBF induce PAX5.
Role of PAX5: Commits cell to B cell lineage, represses alternative lineages, and upregulates B cell-specific genes (e.g., CD19, BLNK).
Rearrangement: V-DJ recombination occurs on the heavy chain.
Allelic Exclusion: Ensures only one allele rearranges at a time.
Rearrangement Outcomes: ~⅔ of rearrangements are non-productive. If the first allele fails, the second allele can rearrange, giving a 55% chance of success.
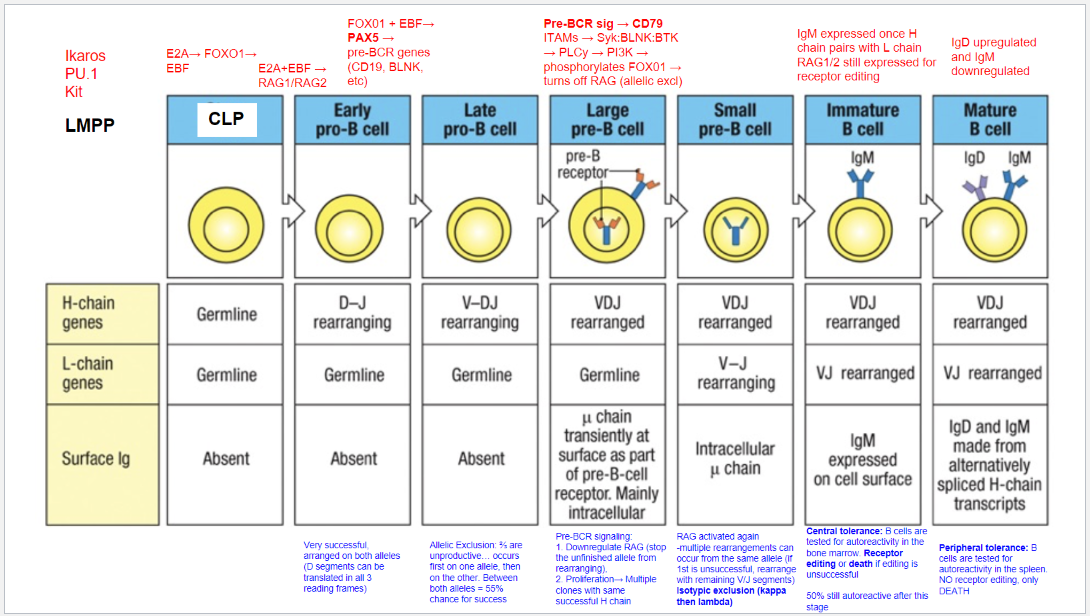
What occurs at the fifth step, the Large Pre-B Cell stage, in B cell development?
Stage: Large Pre-B Cell.
Pre-BCR Signaling: Initiated with CD79 as part of the pre-BCR complex.
Functions of Pre-BCR Signaling:
Allelic Exclusion: Downregulates RAG, stopping further heavy chain rearrangement on the other allele, ensuring only one productive heavy chain.
Proliferation: Drives cell proliferation for clones with identical heavy chains, amplifying cells with a successful heavy chain rearrangement for further development.
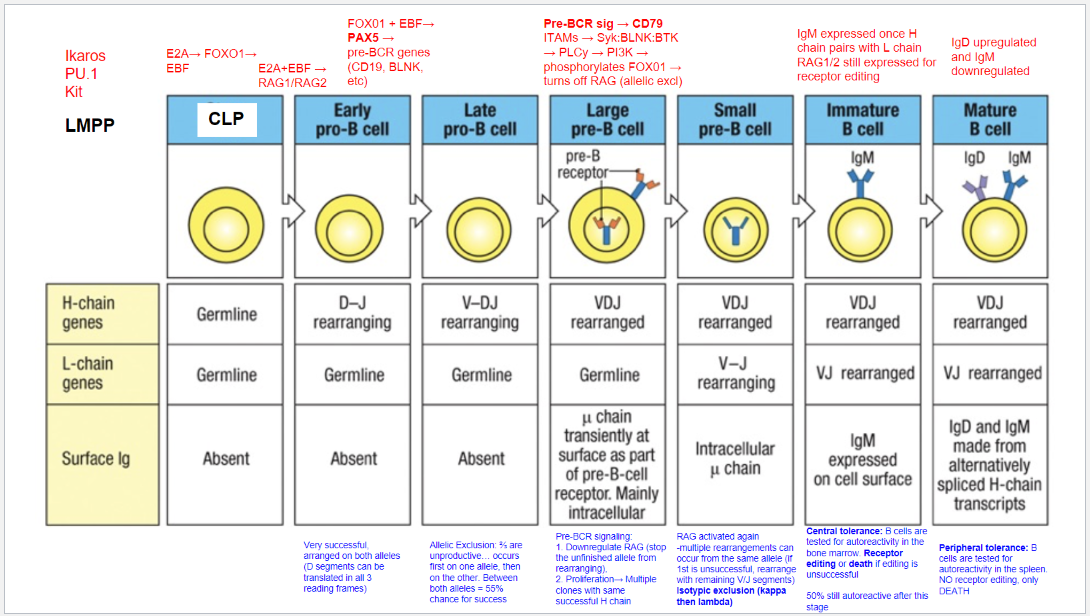
What occurs at the sixth step, the Small Pre-B Cell stage, in B cell development?
Stage: Small Pre-B Cell.
Key Processes:
RAG Reactivation: Enables light chain rearrangement.
Multiple Rearrangement Attempts: Allows retry on the same or other allele if the initial attempt fails.
Isotypic Exclusion:
κ (Kappa) Chain First: Begins with κ light chain rearrangement.
Switch to λ (Lambda) Chain: If κ is unsuccessful, λ chain rearrangement is attempted.
Outcome: Ensures each B cell expresses a unique light chain paired with its heavy chain, creating a single, specific antigen receptor.
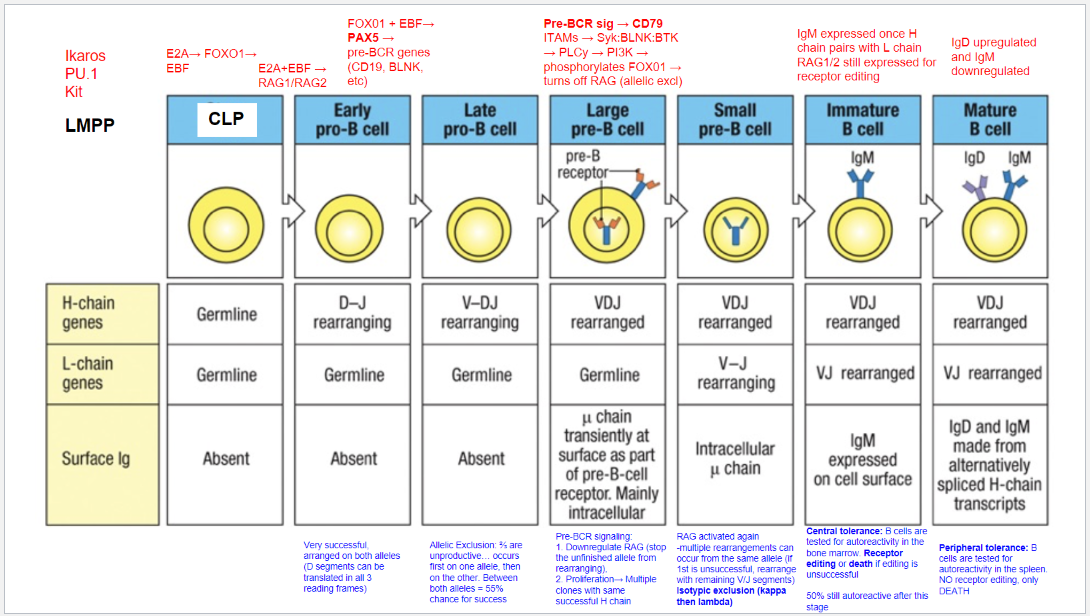
What occurs at the seventh step, the Immature B Cell stage, in B cell development?
Stage: Immature B Cell.
Key Indicators:
Surface IgM Expression: Successful heavy and light chain pairing signifies a functional BCR.
Central Tolerance:
Receptor Editing: Self-reactive cells can rearrange the light chain to avoid autoreactivity.
Apoptosis: Cells that remain autoreactive after editing are eliminated.
Outcome: Ensures mostly non-autoreactive B cells progress to maturity, though ~50% may still display some autoreactivity.
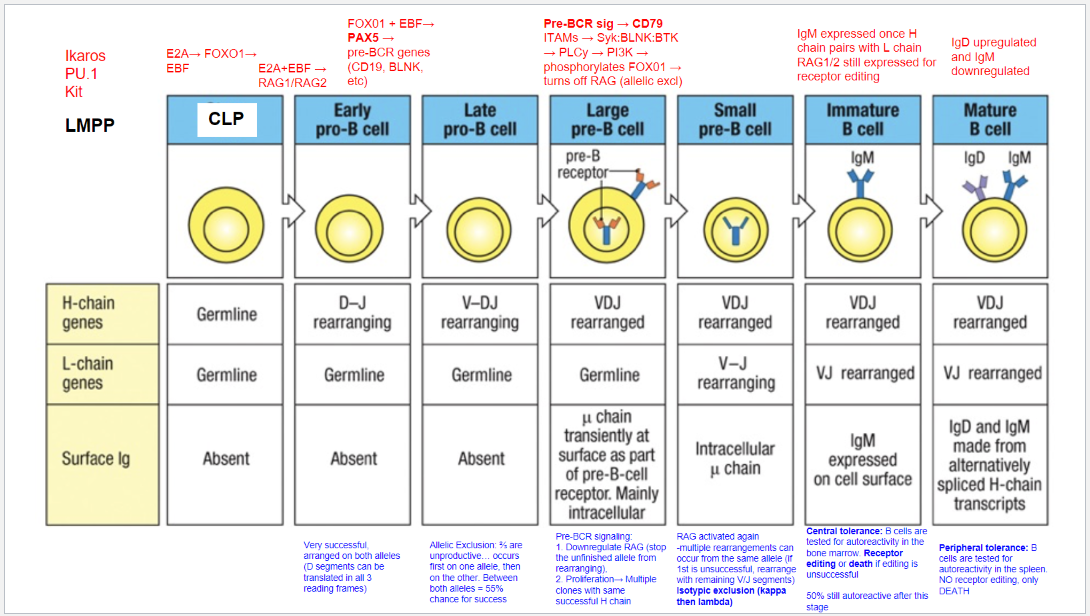
What occurs at the eighth step, the Mature B Cell stage, in B cell development?
Stage: Mature B Cell.
Surface Markers:
IgD Upregulation & IgM Downregulation: Expresses both IgM and IgD via alternative splicing of heavy chain transcripts.
Peripheral Tolerance:
Testing in Spleen: Autoreactive cells undergo apoptosis, with no option for receptor editing.
Outcome: Prevents autoimmunity by eliminating self-reactive mature B cells in the periphery.
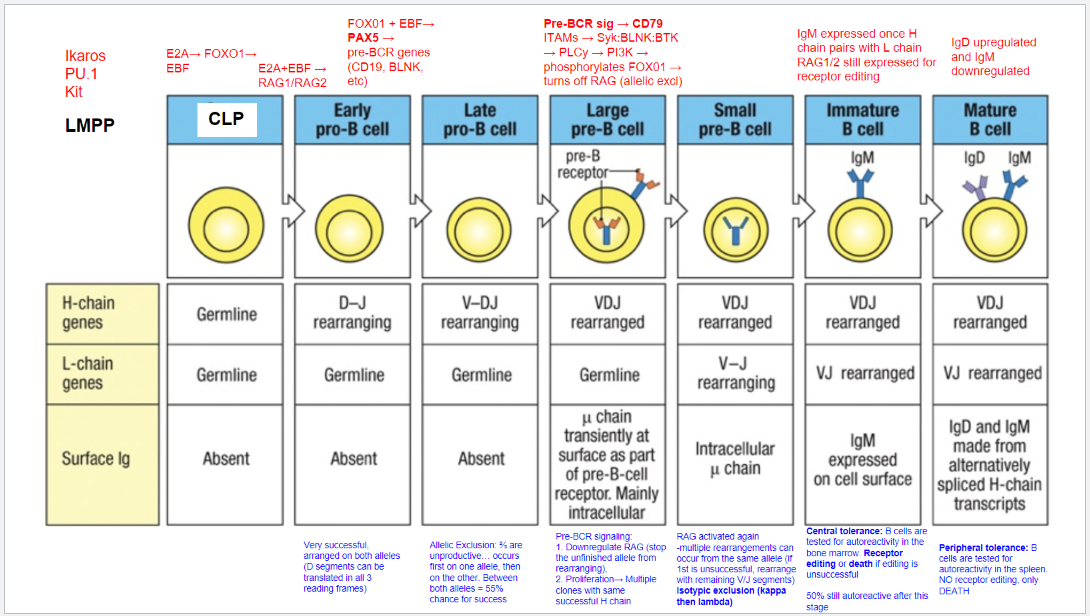
What are the key stages and molecular events in B cell development?
B cell development proceeds through distinct stages, each governed by specific transcription factors and signaling events:
LMPP (Lymphoid-Primed Multipotent Progenitor): Early stage marked by Ikaros, PU.1, and Kit, committing cells to a lymphoid lineage.
CLP (Common Lymphoid Progenitor): E2A, FOXO1, and EBF initiate B cell commitment, activating RAG1 and RAG2 for immunoglobulin gene rearrangement.
Early Pro-B Cell: E2A and EBF continue activating RAG1/RAG2, initiating D-J rearrangement on the heavy chain.
Late Pro-B Cell: FOXO1 and EBF induce PAX5, locking in B lineage and enabling V-DJ rearrangement on the heavy chain. Allelic exclusion ensures only one heavy chain is expressed.
Large Pre-B Cell: Pre-BCR signaling through CD79 downregulates RAG, halts further heavy chain rearrangement, and drives proliferation of cells with a successful heavy chain.
Small Pre-B Cell: RAG is reactivated for light chain rearrangement. Isotypic exclusion occurs with κ and λ light chains, ensuring a single light chain pairs with the heavy chain.
Immature B Cell: With a complete BCR (IgM), cells undergo central tolerance in the bone marrow. Self-reactive cells may attempt receptor editing or undergo apoptosis.
Mature B Cell: Final maturation includes IgD upregulation alongside IgM and peripheral tolerance testing in the spleen. Autoreactive cells at this stage undergo apoptosis.
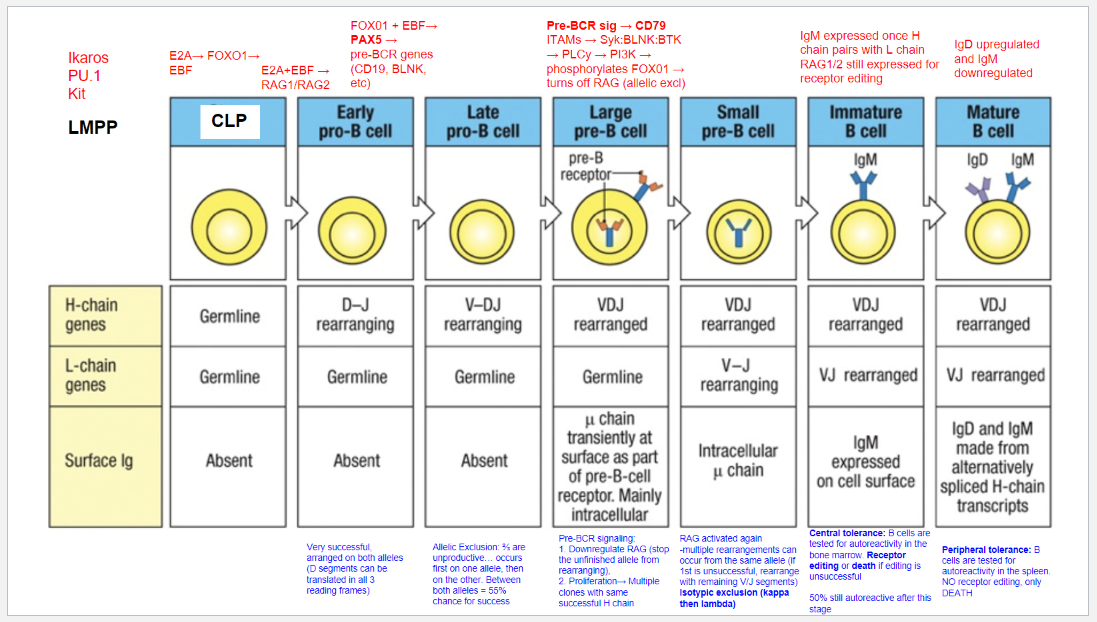
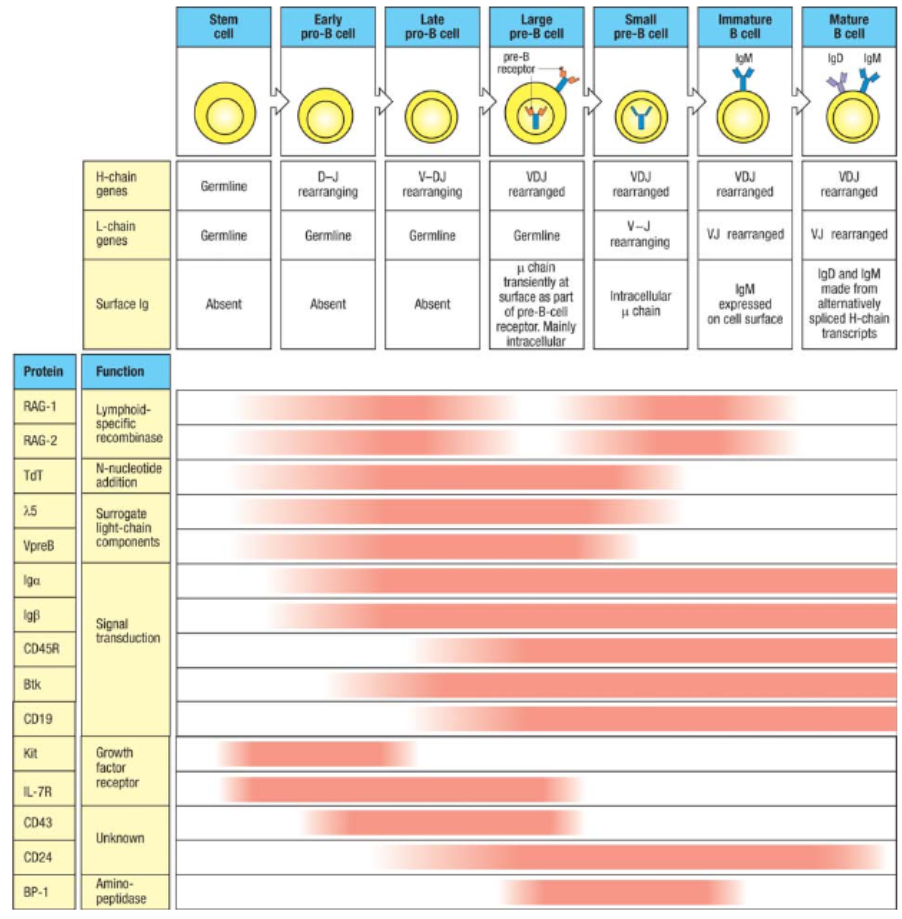
What are the key stages and rearrangements in B cell development as shown in Figure 8.5?
Early Pro-B Cell: Heavy chain gene rearrangement begins with D to J joining on the heavy chain. No functional μ (mu) protein is expressed at this stage.
Late Pro-B Cell: V to DJ rearrangement occurs on one chromosome. If unsuccessful, rearrangement attempts on the second chromosome. A successful rearrangement results in μ chain production.
Large Pre-B Cell: The μ chain pairs with surrogate light chains (λ5 and VpreB) to form the pre-B cell receptor (pre-BCR). Signaling through Igα and Igβ halts further heavy chain rearrangement, enforcing allelic exclusion, and drives cell proliferation.
Small Pre-B Cell: Light chain gene rearrangement begins with V to J joining. Multiple rearrangements on the same or other allele can occur if needed.
Immature B Cell: Successful light chain rearrangement produces a complete IgM molecule, which is expressed on the cell surface, signaling the cell to stop further light chain rearrangements.
Mature B Cell: After passing tolerance checks, the cell expresses both IgM and IgD on its surface, indicating readiness to function in the immune response.
Each stage involves critical rearrangements and checkpoints to ensure the production of functional and self-tolerant B cells.
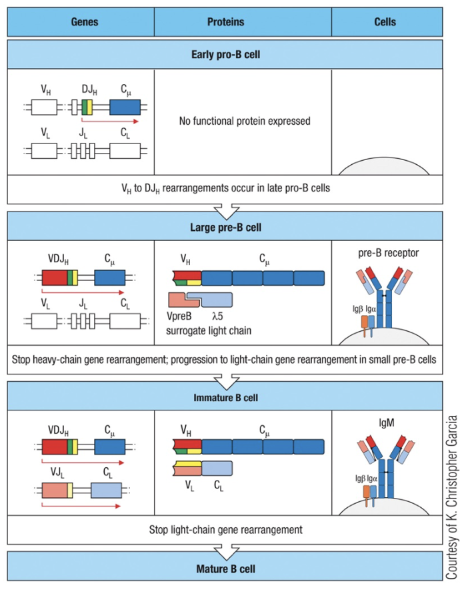
How can nonproductive light chain gene rearrangements be rescued in B cell development?
In B cell development, if the initial V-J rearrangement of the light chain gene is nonproductive, the cell can attempt additional rearrangements to rescue the process.
First Nonproductive Join: If the first V-J recombination fails, another V segment can join with a different J segment, removing the out-of-frame join.
Repeated Attempts: This process can occur up to 5 times on each chromosome, maximizing chances for a productive rearrangement.
Switch to Lambda Chain: If all attempts at kappa chain rearrangement fail, the cell can switch to rearrange the lambda chain.
This mechanism greatly enhances the likelihood of producing a functional light chain, ensuring successful B cell maturation.
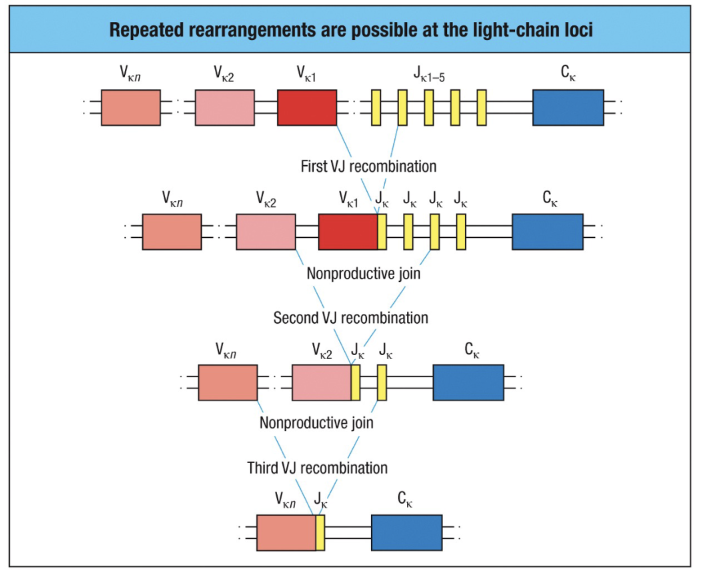
What happens to immature B cells in the bone marrow when they encounter self molecules?
Central Tolerance Testing in bone marrow ensures B cells are non-autoreactive:
No Self Reaction:
B cells mature normally.
Migrate to peripheral lymphoid tissues.
Express both IgM and IgD and become mature, recirculating B cells.
Self-Reaction with Multivalent Self Molecule:
Receptor Editing:
RAG proteins are reactivated for further light chain rearrangement.
If a non-autoreactive receptor forms, the B cell continues development.
Apoptosis (Clonal Deletion):
If receptor editing fails, the cell undergoes apoptosis.
Key Mechanism: Receptor editing predominantly ensures central tolerance, preventing autoimmunity by correcting or eliminating self-reactive B cells.
Defects in this process can lead to autoimmune diseases.
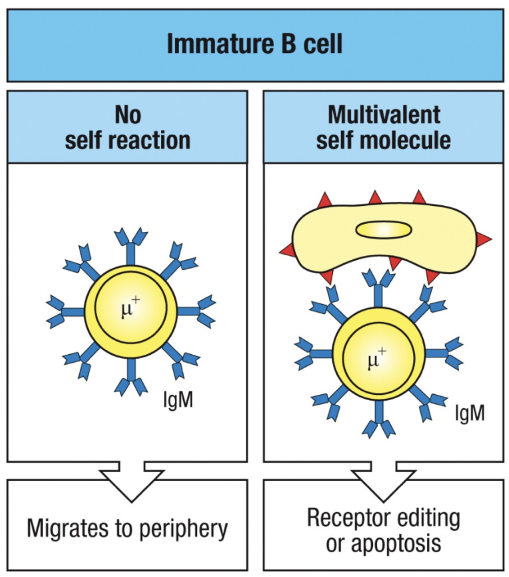
How does receptor editing rescue self-reactive B cells?
When a developing B cell's IgM receptor binds strongly to self-antigens, receptor editing may rescue the cell:
Self-Antigen Recognition: Strong binding of IgM to a multivalent self-antigen signals self-reactivity, leading to a halt in B cell development and a decrease in surface IgM expression.
Continued RAG Expression: RAG proteins remain active, allowing additional light chain gene rearrangements.
New Light Chain Expression: A new light chain is produced, potentially altering the receptor's specificity.
Outcome:
Non-Self-Reactive Receptor: If the new receptor no longer reacts with self-antigens, the B cell resumes development, migrates to peripheral tissues, and matures.
Persistent Self-Reactivity: If the receptor remains self-reactive, the B cell may attempt further rearrangements. If unsuccessful, the cell undergoes apoptosis, known as clonal deletion.
This receptor editing process is crucial for maintaining central tolerance and preventing autoimmunity.
What happens to transitional B cells in the periphery if they encounter self-antigens?
Transitional B cells in the periphery undergo peripheral tolerance:
Self-Antigen Encounter: If the B cell receptor (IgM) strongly binds a multivalent self-antigen, the B cell receives a signal leading to apoptosis, eliminating the self-reactive cell.
No Self-Reaction: If no strong self-antigen interaction occurs, the transitional B cell continues maturing. It upregulates surface IgD, becoming a mature B cell capable of circulating in peripheral lymphoid organs.
This process ensures self-reactive B cells are removed, preventing autoimmunity and maintaining tolerance outside the bone marrow.