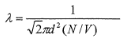Physical Chemistry - Exam 2 Statements
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Le’ Chatelier Principle
If a system at equilibrium is disturbed, it shifts to counteract the change.
Overall order of a reaction
Sum of the exponents in the rate law, indicating the reaction's dependence on reactant concentrations.
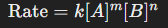
Transition state theory
Reactions pass through a high-energy transition state before forming products.
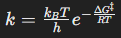
Activation energy
The minimum energy required for a reaction to occur.
Potential energy surface
Graph showing how potential energy changes with atomic or molecular positions during a reaction.
Stead-state approximation
Assumes the concentration of intermediates remains constant during a reaction.
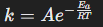
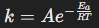
Arrhenius equation
Describes how the rate constant depends on temperature and activation energy.
Half-life of a reaction
Time taken for half of the reactants to be converted into products.

Mean free path
Average distance a molecule travels before colliding with another.