Specific latent heat: Particle model of matter: Physics: GCSE (9:1)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
15 Terms
Latent heat
The energy required or released when a substance changes state

What temperature do changes of state occur at?
At fixed temperatures
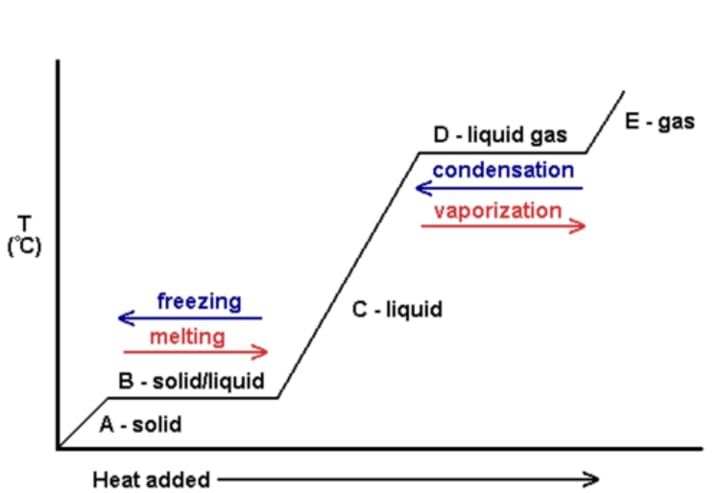
Specific latent heat
The amount of energy required to change the state of one kilogram (1 kg) of the substance with no change in temperature
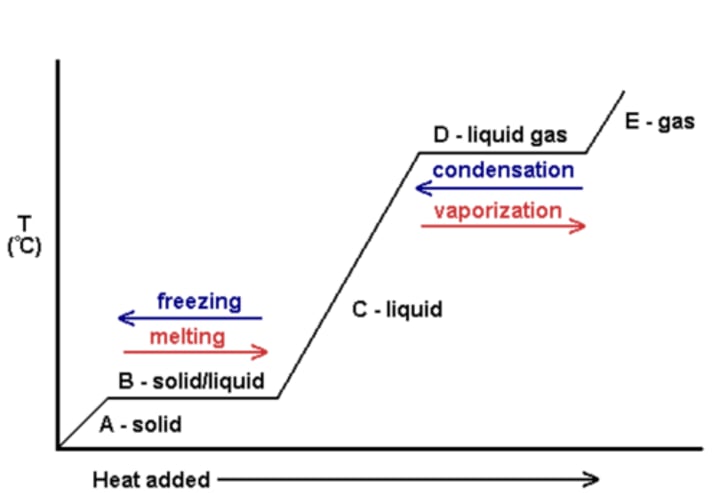
Specific latent heat of fusion
The energy required when a substance changes from a solid to a liquid (or the energy released when a substance changes from a liquid to a solid)
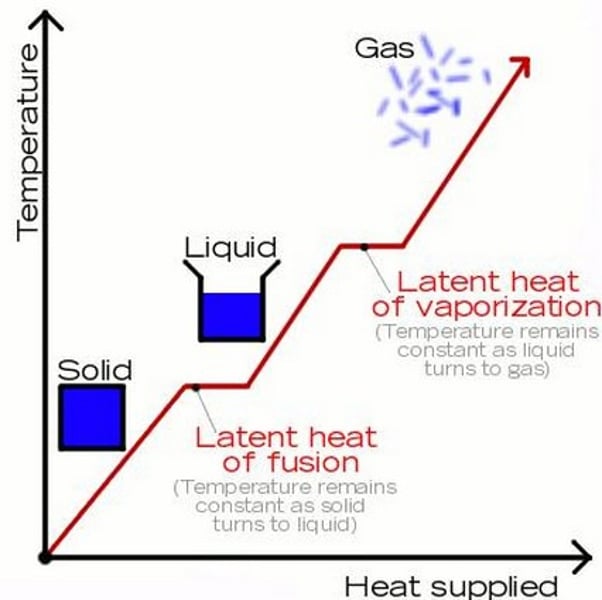
Specific latent heat of vaporisation
The energy required when a substance changes from a liquid to a gas (or the energy released when a substance changes from a gas to a liquid)
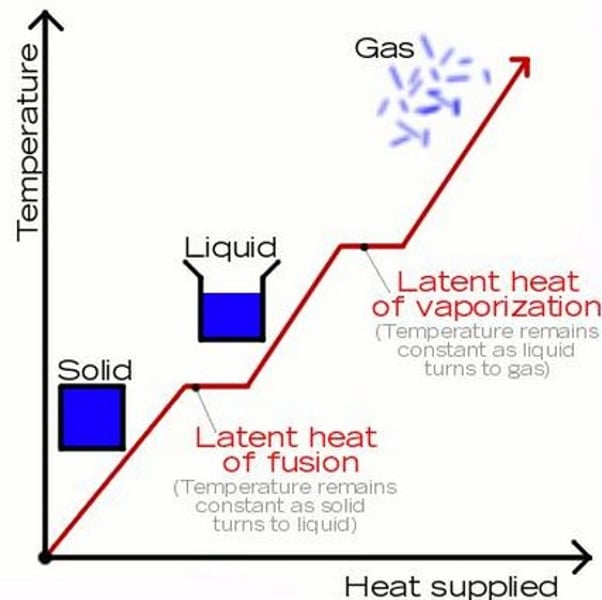
E = mL
The equation linking energy (for a change of state), mass and specific latent heat
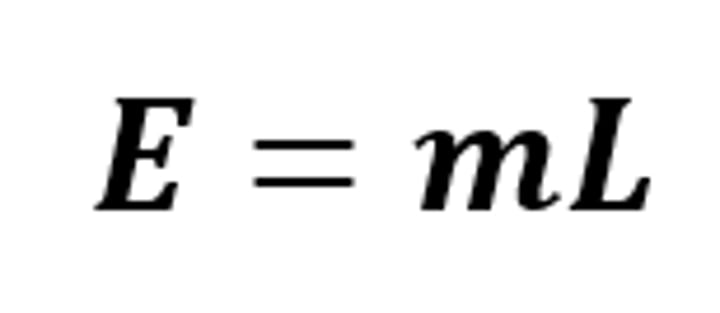
E
The symbol for energy
m
The symbol for mass
L
The symbol for specific latent heat
Joules (J)
The SI unit for energy and work

Kilograms (kg)
The SI unit for mass
Joules per kilogram (J/kg)
The SI unit for specific latent heat
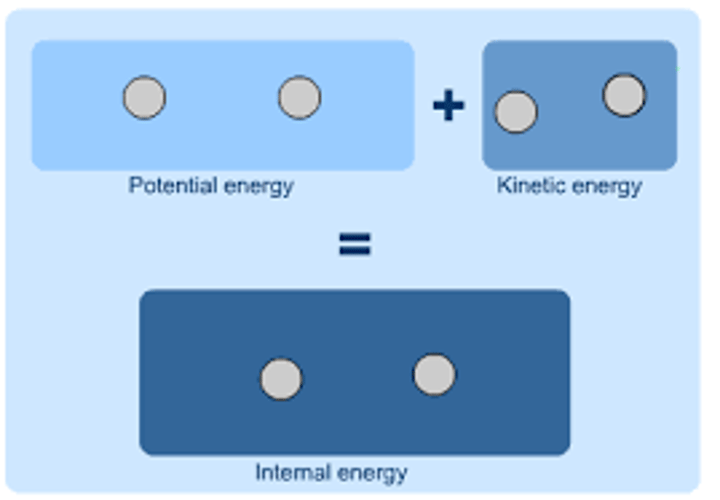
How does changing the internal energy of a substance affect it?
The substance will change temperature or change state
What does the specific heat capacity of a substance tell us ?
The energy required (per kilogram) for the substance to change temperature
What does the specific latent heat of a substance tell us ...
The energy required (per kilogram) for the substance to change state