Final Test!
0.0(0)
Card Sorting
1/108
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
1
New cards
NH₄⁺
ammonium
2
New cards
CO₃²⁻
carbonate
3
New cards
ClO₃⁻
chlorate
4
New cards
ClO₂⁻
chlorite
5
New cards
ClO₄⁻
perchlorate
6
New cards
ClO⁻
hypochlorite
7
New cards
OH⁻
hydroxide
8
New cards
NO₃⁻
nitrate
9
New cards
NO₂⁻
nitrite
10
New cards
PO₄³⁻
phosphate
11
New cards
PO₃³⁻
phosphite
12
New cards
H₂PO₄⁻
dihydrogen phosphate
13
New cards
HPO₄²⁻
hydrogen phosphate
14
New cards
SO₄²⁻
sulfate
15
New cards
SO₃²⁻
sulfite
16
New cards
HSO₄⁻
hydrogen sulfate (or bisulfate)
17
New cards
HCO₃⁻
hydrogen carbonate (or bicarbonate)
18
New cards
CN⁻
cyanide
19
New cards
BrO₄⁻
perbromate
20
New cards
BrO₃⁻
bromate
21
New cards
BrO₂⁻
bromite
22
New cards
BrO⁻
hypobromite
23
New cards
IO₄⁻
periodate
24
New cards
IO₃⁻
iodate
25
New cards
IO₂⁻
iodite
26
New cards
IO⁻
hypoiodite
27
New cards
CH₃COO⁻ (or C₂H₃O₂⁻)
acetate
28
New cards
1
Mono
29
New cards
2
Di
30
New cards
3
Tri
31
New cards
4
Tetra
32
New cards
6
Hexa
33
New cards
7
Hepta
34
New cards
8
Octa
35
New cards
9
Nona
36
New cards
10
Deca
37
New cards
5
Penta
38
New cards
Bond Order
Number of bonds between 2 atoms
39
New cards
increases, decreases
As the bond order ____ the bond length _____
40
New cards
increases, increases
As the bond order ____ the bond energy _____
41
New cards
Formal Charge
(# of valence)-(#of bonds)-(# of lone electrons)=
42
New cards
Linear, 180
2 ED, 0 Non-bonding pairs
43
New cards
Trigonal Planar, 120
3 ED, 0 Non-bonding pairs
44
New cards
Bent,
3 Ed, 1 Non-bonding pair
45
New cards
Tetrahedra,l 109.5
4 ED, 0 Non-bonding pairs
46
New cards
Trigonal Pyramidal,
4 ED, 1 Non-bonding pairs
47
New cards
Bent, << 109.5
4 ED, 2 Non-bonding pairs
48
New cards
Trigonal Bipyramidal, 120, 90
5 ED, 0 Non-bonding pairs
49
New cards
SeeSaw
5 ED, 1 nonbonding pair
50
New cards
T-shape
5 ED, 2 nonbonding pairs
51
New cards
Linear
5ED, 3 nonbonding pairs
52
New cards
Octahedral 90
6ED, 0 non-bonding pairs
53
New cards
Square Pyramidal
6ED, 1 non-bonding pair
54
New cards
Square Planar
6ED, 2 non-bonding pairs
55
New cards
Central Atom
(A)BU
56
New cards
# of bonding ED
A(B)U
57
New cards
# of nonbonding/unbonding Eds
AB(U)
58
New cards
London Dispersion Forces
In “Induced dipole”, two particles will be attracted to each other weakly briefly. All molecules have this. Weakest IMF
59
New cards
Dipole-Dipole
Happens in polar molecules. Stronger than London, weaker than hydrogen
60
New cards
Hydrogen Bonding
Occurs when hydrogen is bonded to Nitrogen, Oxygen, or Fluorine. Strongest IMF within bonding of two nonmetals.
61
New cards
The higher the melting point
The stronger the IMF...
62
New cards
Ion-Dipole
Strongest IMF, occurs when a metal is bonded with a nonmetal.
63
New cards
sp
Linear Hybridization
64
New cards
sp^2
Trigonal Planar Hybridization
65
New cards
sp^3
Tetrahedral Hybridization
66
New cards
sp^3d
Trigonal Bipyramidal Hybridization
67
New cards
sp^3d^2
Octahedral Hybridization
68
New cards
1 sigma
Single Bond
69
New cards
1 sigma 1 pi
Double Bond
70
New cards
10^12
tera (T)
71
New cards
10^9
Giga (G)
72
New cards
10^6
Mega (M)
73
New cards
10^3
Kilo (k)
74
New cards
10^-1
deci (d)
75
New cards
10^-2
cent (c)
76
New cards
10^-3
milli (m)
77
New cards
10^-6
micro (u)
78
New cards
10^-9
nano (n)
79
New cards
10^-12
pico (p)
80
New cards
Intensive
Boiling Point, color, temperature, luster, hardness
81
New cards
Extensive
volume, mass, size, weight, length
82
New cards
isotopes
nuclides with the same atomic number but different mass number
83
New cards
unstable
All nuclei z> or equal to 83 will be ____, as well as Te (43)and Pm (61)
84
New cards
stable
Nuclides are most likely to be ____ if the isotope and atomic mass are close.
85
New cards
stable.
Nuclei with even numbers of protons and/or neutrons are more _____
86
New cards
c=speed of light=wavelengthxfrequency
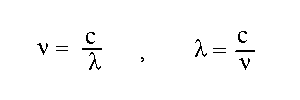
87
New cards
Energy Equation
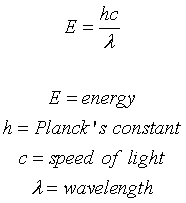
88
New cards
Cr [Ar] 4s^1 3d^5, Cu [Ar] 4s^1 3d^10
orbital anomalies
89
New cards
has all electrons paired
diamagnetic
90
New cards
has unpaired electrons
paramagnetic
91
New cards
Trends
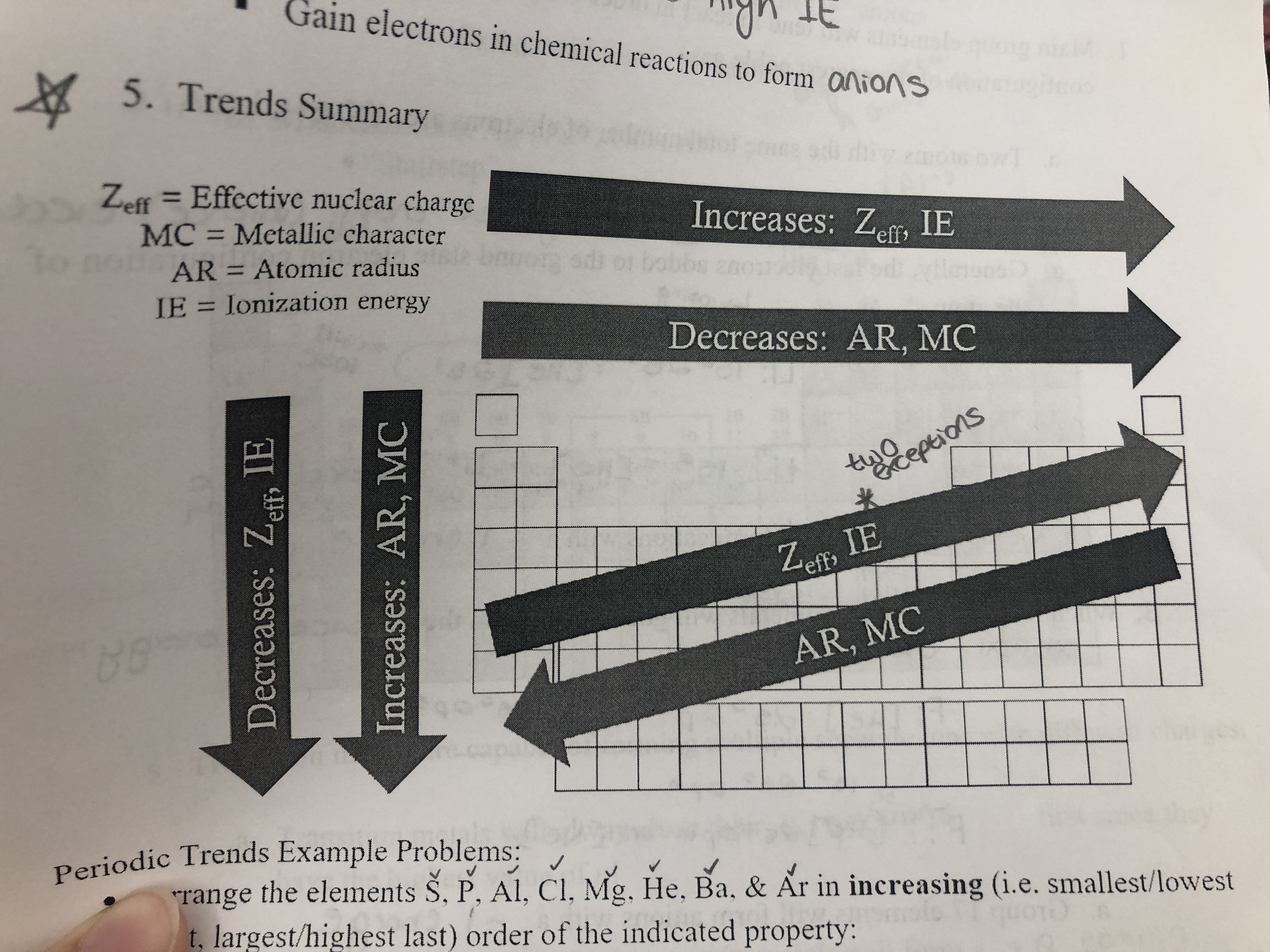
92
New cards
Ionization Energy Exceptions
3A and 6A elements have lower IE than the 2A and 5A elements respectively
93
New cards
Group 1 Elements (Li, Na, K, Rb, Cs) and NH4, NO3, C2H3O2, ClO3
Always soluble
94
New cards
Most Group 17 anions (Cl, Br, I, F): Except with Ag, Hg2+, Pb2+, SO42-: Except with Ag, Hg2+, Pb2+, Ca2+, Sr2+, Ba2+
Usually soluble
95
New cards
CO32-, PO43-, CrO42-, S2-, (M(OH): Expect with Ba(OH)2, Sr(OH)2, Ca(OH)2
Usually insoluble
96
New cards
HCl, HBr, HI, HNO3, HClO4, HClO3, H2SO4
Strong Acids
97
New cards
LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, Ba(OH)2
Strong Bases
98
New cards
Oxidation
Loss of electrons by a substance
99
New cards
Reduction
Gain of electrons by a substance
100
New cards
+1,-1
Hydrogen is __ when paired with a nonmetal, and __ when paired with a metal