Rate of Reaction summary
1/55
Earn XP
Description and Tags
Summary of all topics covered Collision Theory
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
Collision Theory (using bullet points - )
-Must collide with activation energy to be successful (if not particles bounce off each other with no successful collisions so no reaction occurs)
-RATE OF REACTION depends on two things: how frequently and forcefully particles collide.
-if frequency and forcefulness are changed, RATE OF REACTION will change
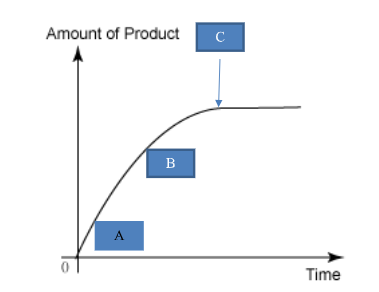
Collision Theory explanations: Label A, B, and C in terms of collision theory
*This is accurate for any fizzy reaction
A: many reacting particles, very frequent collisions
B: fewer reacting particles, less frequent collisions
C: no more reacting particles as one reactant has been used up
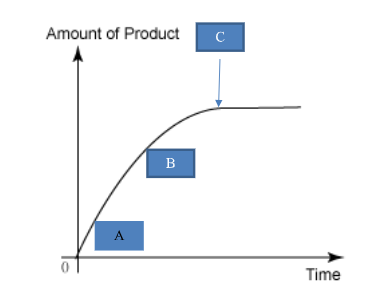
Label A, B and C (not collision theory)
*This is accurate for any fizzy reaction
A: Reaction fastest (steepest gradient)
B: Reaction slowing (less steep gradient)
C: Reaction stops
Rate of Reaction definition
the change in amount of substance per second
To find mean rate
read off graph and divide y/x
To find mean rate using a tangent
Draw tangent, turn into triangle, divide y/x
Gas Syringe Method definition
The gas syringe is used to collect and measure the amount of gas produced during a reaction from a conical flask.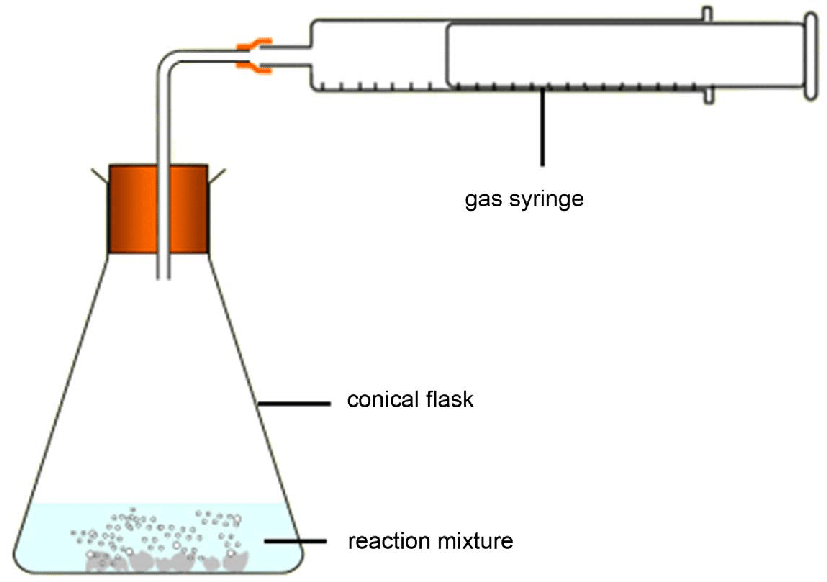
Gas Syringe Method Advantages
Good Resolution, Gases are not released into the room.
Gas Syringe Method Monitoring Options
Either decide time and measure volume or decide volume and measure time or measure at intervals.
Mass Loss method definition
MEASURES GAS: Use scales to measure how much mass is lost with conical flask and cotton wool ball (only allows gas to escape, not liquid).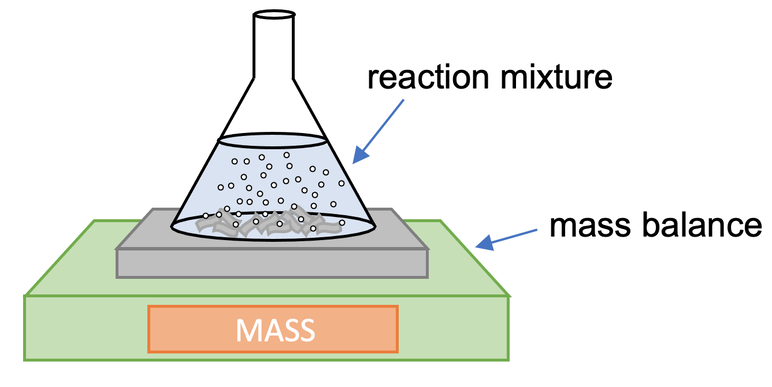
Mass Loss method advantages
Excellent resolution: masses measured to the nearest 0.01g
Mass Loss method disadvantages
Gases are released into the room
Mass Loss method monitoring options
Either choose time and measure mass loss or choose mass loss and measure time. Or measure at regular time intervals.
Monitoring Rate of Reactions that don’t produce gas: DEFINITION
If no gas is produced and reaction produces a precipitate or dark colour, time how long it takes for a cross on piece of paper to disappear.
Conical Flask
Precipitate
A solid formed by change in solution.
What factors affect rate
Surface Area, Concentration, Pressure, Temperature
Repeatable
I do it lots and get the same results
Reproducable
Others do it lots and get the same results
In the order Concentration, Surface Area, Pressure, Temperature how do these factors effect rate?
Concentration: Higher concentration, faster rate of reaction
Surface Area: Larger surface area, faster rate of reaction.
Pressure: Increased pressure, faster rate of reaction.
Temperature: Increased temperature increases rate of reaction.
How is pressure caused?
Gas particles colliding with container walls
When does a reaction stop?
When one reactant has been used up so there are no more collisions.
Independent Variable
What I change
Dependent Variable
What I observe
Controlled Variable
What I keep the same
Explain, in terms of particles, why rate of reaction increases with temperature.
-Temperature increase means particles have more energy
-Particles have activation energy
-More frequent collisions
-More forceful collisions
What does a Catalyst do?
Provides a new route for a reaction with lower activation energy so particles can collide more frequently
particles HAVE NOT got more energy!! ONLY
temperature increase can do this
Who are the best catalysts?
Transition metals (specifically manganese oxide) and their compounds
Advantages of using a catalyst + explanations
Reactions done at lower temperatures
-less non-renewable fossil fuels used
-less CO2 emissions, less global warming
-less SO2 emissions, less acid rain
-less NOx emissions, less acid rain
-less particulates, less global dimming and lung diseases
Sustainable
-less non-renewable sources of metal catalyst used
-less mining of metal catalyst, less habit destruction
Disadvantages of using a catalyst + explanations
Fill up landfill sites on disposal:
-they do not decompose/rot
-can contain toxic poisons that enter environment
Eventually stop working so mining of metal catalysts:
-expensive to mine/extract
-rare metals are very expensive to mine/extract
‘floating head reaction’
RP: Sodium Thiosulphate-HCl Reaction
Important information
-no gas produced in this reaction so we cannot use a gas syringe or a balance to monitor it
-A yellow precipitate of sulphur is produced (S(s))
-we measure time taken for the cross to dissapear
‘floating head reaction’
RP: Sodium Thiosulphate-HCl Reaction
What is it used for?
Any reaction that produces a precipitate or a dark colour
‘floating head reaction’
RP: Sodium Thiosulphate-HCl Reaction
Variables
IV: Concentration of ‘thio’
DV: Time for cross to disappear
CV: Concentration of HCl
Volume of HCl
Total volume of ‘thio’
Constant Temperature
‘floating head reaction’
RP: Sodium Thiosulphate-HCl Reaction
Accuracy
To improve accuracy
-use pipette or burette to measure vol
-use light sensor connected to a data logger to measure when light intensity drops to a certain value (as precipitate forms)
-use water bath set at fixed temp to keep contents in a flask at a constant temperature
Calcium Carbonate (marble) or Magnesium HCl reaction
Methods
-Measure gas volume with a gas syringe
-Measure mass loss using a balance
Calcium Carbonate (marble) or Magnesium HCl reaction
Variables
IV: Concentration of HCl
DV: Time for cross to disappear
CV: Total voume of HCl
Same mass of solid
Constant room temperature
Calcium Carbonate (marble) or Magnesium HCl reaction
Accuracy
To improve accuracy:
-use pipette or burette to measure volume
-use water bath set at fixed temp to keep contents at a constant temp.
How to prove a Catalyst can not be used up
-weigh catalyst
-filter mix
-dry catalyst
-reweigh cat.
Endothermic
reactants absorb heat energy from the surroundings to form products
Exothermic
releases heat, causing the temperature of the immediate surroundings to rise
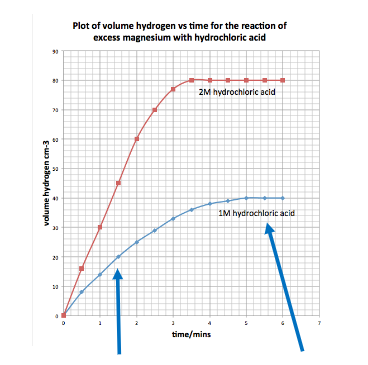
Volume/Mass Loss vs time graph
Lower Concentration: Slower (less steep) and less gas produced
Halving concentration: halves volume/ mass gas produced
Concentration mathematical link
-Rate directly proportional to concentration (rate ∝ concentration)
e.g if doubling conc doubles rate
twice as many reacting particles
twice as many frequent collisions
Temperature mathematical link
-Rate is NOT directly proportional to temperature
-Rate approximately doubles per 10 degree rise
More diluted solution means
Less gas produced (less steep line, finishes later)
Change in surface area
Does not change how much gas is produced, just gives a much faster reaction
Surface area mathematical link
-rate is directly proportional to surface area
-e.g doubling surface area doubles rate
twice as many reacting solid particles available
twice as many frequent collisions
Pressure mathematical link
-rate directly proportional to pressure
-e.g doubling pressure doubles rate
same number of particles in half the volume
twice as many frequent collisions
Straight line through origin
Directly proportional
Change in temperature
Does not change amount of gas produced, just gives a faster reaction
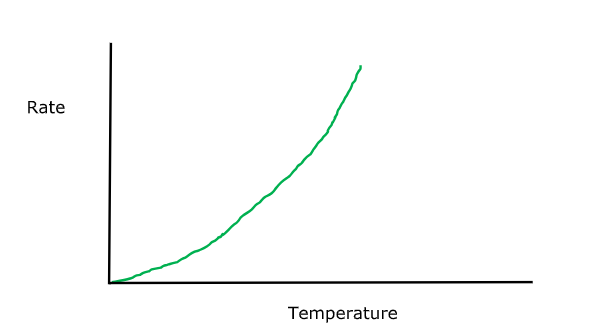
Change in temperature graph
Not straight line through the origin:
Rate is not directly proportional to temperature
Pattern is called ‘exponential’
‘exponential’
increase one thing by a certain amount each time (eg 10 degrees)
other ‘thing’ increases by more and more each time
(eg by 2, then 7, then 25 etc)
The most common reactions that produce gas
Adding metal to an acid to produce H2 gas
for example:
Magnesium + Hydrochloric Acid → Magnesium Chloride + Hydrogen
Adding a carbonate to an acid to produce CO2 gas
for example:
Calcium Carbonate + Hydrochloric Acid → Calcium Chloride + Carbon Dioxide + Water
The rate of any reaction that produces a gas
can be measured by mass loss or gas volume methods
A successful collision means
bonds are broken in reactants
the particles separate from each other
they combine differently
new bonds form between them to make the products
Making catalysts even better
-Powdered Catalysts: Large Surface Area, Reactions go even faster
-Nanocatalysts: Huge surface area to volume ratio (huge surface area compared to volume)
Catalysts and rate
Hydrogen Peroxide solution (aq) breaks down very slowly. Can be sped up using a catalyst.