RENR 445 soil fertility midterm 2
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
117 Terms
What type of nitrogen makes up 78% of the atmosphere?
N2 gas (inert nitrogen)
What are the major sources of reactive N (N available to biota)?
inorganic fert produced via Haber-Bosch synthesis
biologically fixed N
atmospheric deposition of N that previously volatized elsewehere
release of N from soil organic matter mineralization
Total nitrogen (TN): total organic nitrogen (TON) + inorganic nitrogen. Where is organic nitrogen and inorganic nitrogen found? Where is Organic N in the soil profile?
ON is found in SOM - 80% of N is ON
inorganic (mineral) N (NH4+ and NO3-) accounts for 1-2% of soil TN and is altered by fertilization
Organic N in soil profile is found nearer to the surface, down to bout 40 cm
Total N in AB soils
more N in fine soil (vs coarse)
more N in luvisolic vs chernozemic soil
What is soil organic nitrogen (SON) comprised of?
amide, amine, and hertocyclic N bonds . 90% SON is sorbed to soil minerals or protected within aggregates
SON is also present in:
particular organic matter (POM; 1-4%), which is a transitory pool of decomposing organic material
dissolved organic nitrogen (DON; 0.1-2%): the soluble N in the soil solution
How is dissolved organic nitrogen (DON) divided?
high molecular weight (HMW) —> proteins
Low molecular weight (LMW) —> amino acids, peptides
What is depolymerization in the context of HMW DON?
HMW DON require extracellular enzume mitigated degradation to LWM DON
LWM DON can be taken up by microbes and plant roots
What is the main source of N for most crops?
inroganic N
- ammonium (NH4+)
nitrate (NO3-)
What is the preferred form of N for plant uptake?
nitrate
analyze and explain the transformations of N in the soil and how those transformations are controlled
industrial fixation
industrial conversion of molecular N to ammonia NH3
atmospheric fixation
lightning and photochemical conversion of molecular N to nitrate N2 —> NO3-
biological fixation
prokaryotic conversion of molecular N to ammonia
N2 —> NH3
plant acquisition
plant absorption and assimilation of ammonium or nitrate
immobilization
microbial absorption and assimilation of ammonium or nitrate
ammonification
bacterial and fungal catabolism of SOM to ammonium
nitrification
bacterial oxidation of ammonium to nitrite and bacterial oxidation of nitrite to nitrate
denitrification
bacterial conversion of nitrate to nitrous oxide and molecular nitrogen
mineralization
bacterial and fungal catabolism of SOM to mineral N through ammonifcation
volatilization
physical loss of gaseous ammonia to the atmosphere
nitrate leaching
physical flow of nitrate dissolved in gw out of the topsoil and eventually into the oceans
N2 fixation
biological nitrogen fixation
lighting
haber-bosch process
N2 fixation by lighting
N2 + O2 —NOx
NOx + H2O —> HNO2 or HNO3
falls to the surface in rain
source of NO3-
haber-nosch process
developed in 1900s by haber, modified in 1912 by bosch
uses high pressure, N2 and H2 to form NH3
biological N2 fixation
carried about by a group of prokaryotes (Diazotrophs) using the enzyme nitrogenase
requires a large energy investment: 16 moles of ATP for each mole of N
symbiotic and nonsymbiotic N2 fixation
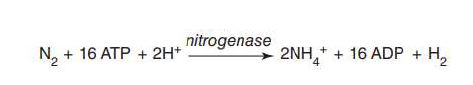
c N2 fixation
symbiotic biological N2 fixation
species-species association in root nodules between Rhizobium species and legumes, also Frankia and some non-legume trees
legumes (alfalfa, beans, chickpeans, soybeans, peas, lentils)
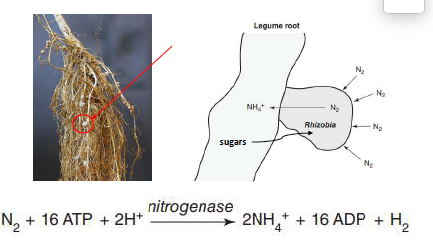
plants exchange energy (sugars and CHO) for fixed N from bacteria
meets 25-90% of plant N requirements
can meet some of the N requirements of subsequent plants through minerlization oh host plant residue
depends on the quantity of N2 fixed and the amount of reside returned
what is symbiotic biologcal N2 fixation affected by:
pH: low pH impairs rhizobial activity
nitrate: preferred alternative to biological fixation as a source of N for the host plant
other limiting factors (water, other nutrients)
species: forages > pulse crops
nonsymbiotic biological N2 fixation
free-living or associative
non-specific association between micro-organisms and plants
ex: cyanobacteria and rice in paddy soils
fixation rates are much lower than those of symbiotic bacteria
atmospheric N desposition
source of reactive N
natural and anthropogenic sources of atmospheric N deposition:
lighting, vocanoes
combustion of fossil fuels, industrial processes
agricultural practices
biomass burning
NOx, HNO3, NH3, NH4+, NO3-
There are two ways of atmospheric deposition which are:
wet deposition
rain, snow
dry deposition
dust
gaseous adsorption
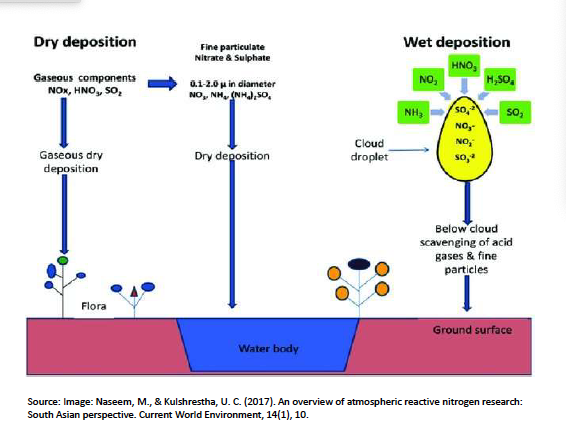
What is cloud scavenging?
the condensation of water vapour on aerosol particles during the formation of cloud droplets
Below cloud scavenging: raindrops dissolve particles during their fall
spatial patterns of N depositition?
need to look into more
depends on emission sources, atmospheric transport, prec. patterns
more in industrial areas and agricultural hotspots
environmental impacts of Atm N deposition
reduces biodiversity, contributes to eutrophication, acidification, declining air and water quality
components of SOM
~50% SOC, 5% SON
What is SOM? SOM Sources?
sources:
aboveground biomass
belowground biomass
soil organisms
microbes us OM decomposition as a source of energy and nutrients
SOM impacts:
nutrient supply
soil structure
available water holding capacity
reduces erosion
biological activity
nutrient retention and buffering capacity
C sequestration
pollutant buffering
SOM decomposition
performed by hetertrophic bacteria to derive energy
hetertrophs cannot produce their own food, they extract energy from organic carbon and nitrogen (plant litter, SOM)
hetertrophs oxidize C and N-rich compounds to release chemical energy required for metabolic functioning
decomposition of SOM depends on extracellular enzymes produced by microorganisms
rate of SOM decomposition is dependent on:
temp
soi moisture
microbial community composiiton (oligotrophs vs copiotrophs)
pH
In order to be transported to microorganisms and transferred across microbial membranes, organic substrates must be in what form?
dissolved form
mineralization
organic N —> mineral N via biological oxidation
performed by hetertrophic soil microorganisms
2 types of mineralization
aminization, ammonification
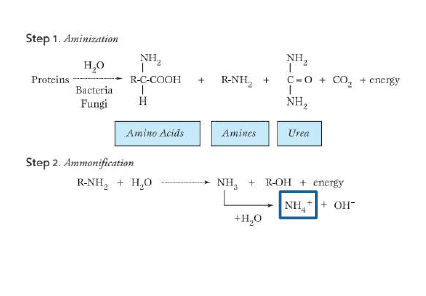
aminization
macromolecules of organic C —> amino acids, amines, urea
ammonification
amino acids, amines, urea —> NH4+
rates of mineralization dependent on:
quantity of substrate
quality of substrate
soil management practices
temp
soil moisture
aminization and ammonifcation chemical process
immobilization
mineral nitrogen —> organic nitrogen via microbial assimilation
reverse of mineralization
Mineralization and immobilization
depends on the C:N ratio of the substrate vs the microbe
hetertrophic microbes seek to maintain a C:N ratio of 8:1
during decomposition, microbes oxidize ~50% of substrate to CO2
carbon use efficiency (CUE)
therefore, if initial C:N ratio is >16:1, then microbes take up mineral N from the soil (immobilization)
but products of substrate decomposition will have a smaller C:N ratio because 50% of C has been oxidized
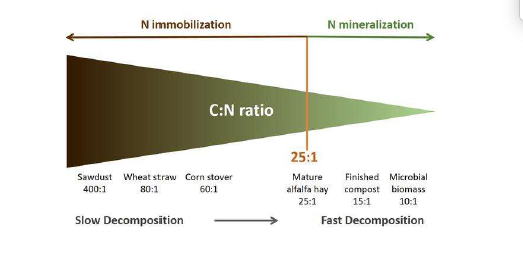
the lower the initial C:N ratio of the residues, the more rapid the initial decomposition process
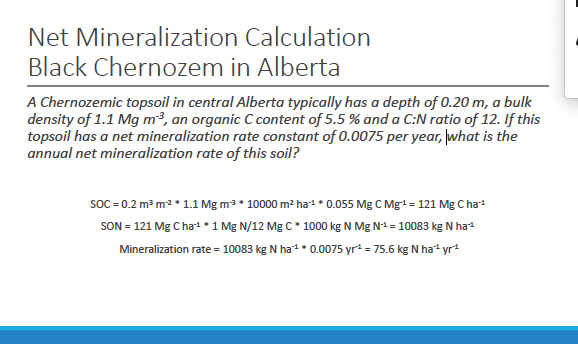
net mineralization calculation
how to build-up and maintain SOM
reduced or no till
cover crops
clover, fall rye, radish, ryegrasses, alfalfa, hairy vetch, winter wheat,
fertilizer, manure
soil health
the continued capacity of a soil to function as a vital living ecosystem that sustains plants, animals, and humans
characteristics of a healthy soil affected by SOM
sufficient depth
good water storage and drainage
sufficient (but not excess) nutrients
large pop. of beneficial organisms
resistance to degradation
resilience in unfavorable conditions
common soil constraints affected by SOM
soil compaction
poor aggregation
low water and nutrient retention
emerging view vs previous view of SOM persistence
emerging view: biological, physical and chemical transformation processes convert SOM into products that form associations with minerals
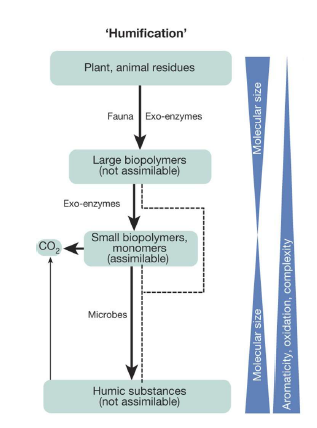
previous: ‘humification’ creates large, complex, recalcitrant ‘humic substances’ to make soil ‘humus’
the existence of humic substances has not been verified by direct measurements
soil humification model - humification
further transformation of initial decomposition products into large, dark-colored compounds
results in macromolecules that are rich in C and N (‘humic substances’
resistant decomposition and are stable
Soil Continuum Model
SOM as a continuum
plants and fauna are broken down via decomp
a continuum exists from large, energy-rich compounds, to small, energy-poor compounds
excludes any secondary s
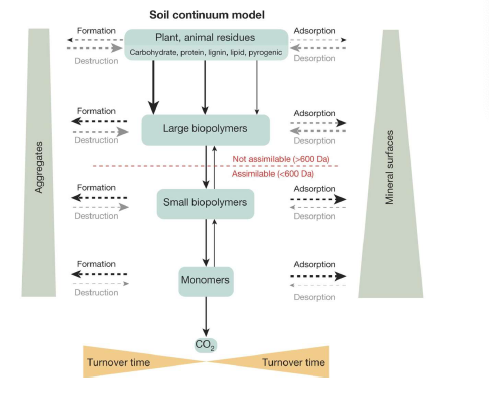
ynthesis of ‘humic substances’
SOM protected by:
physical protection
chemical protection
microbial processing increases polar groups
polar groups= sorption onto soil mineral surfaces and inclusion in aggregates
spatial heterogeneity
molecular diversity
temporal mismatch
fates of ammonium:
immobilized
adsorbed to surfaces and soil particles
fixed in expanded latices of clay minerals
nitrification
volatilization (N loss)
soil colloids (reactive fraction)
finer size fractions of the soil (clay and OM)
most chemically active portion of soil
<2.0 micrometers
high SA
colloidal properties of soil (imparted by clays and OM)
nutrient retention
shrink/swell
water holding capacity
buffering capacity
cation exchange capacity
the number of centimoles of positive charge that can be adsorbed per unit mass
determined by the amounts of different colloids
cations commonly held:
NH4+, Ca2+, Mg2+, K+, H+, Al3+
cation exchange
cations in soil solution exchange with cations attached to colloids
NH4+ adsorption
results from the charge on clay minerals
Al3+>H+>Mg2+>K+ = NH4+>Na+
NH4+ adsorption is affected by:
CEC
concentration in soil solutions
presence of other cations
pH
NH4+ fixation
ammonium replaces interlayer cations in expanded lattices of clay minerals
slow equilibrium with echangeable NH4+
affected by:
wetting-drying, freezing-thawing expands lattices, promotes fixation
K+: closes lattices, reduces fixation
nitrification
2 steps
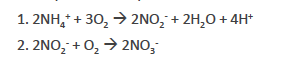
performed by chem0autotrophic bacteria
generation of H+ causes soil acidification over time
sustained use of NH4+ fertilizers requires periodic liming (CaCO3)
the repeated addition of ammonium-based fertilizers causes nitrification rates to be higher than under natural conditions. Nitrification —> soil acidification
factors affecting nitrification
supply of NH4+
soil pH
O2 supply
soil moisture
soil temp
denitrification
carried out by facultative anaerobic bacteria and fungi
performed because microbes require a TEA
removes Nr from soil and returns N2 to the atmosphere
NO3- used as TEA

factors affecting dentrification
OM
water comtent
pH
temp
No3-
plant growth
N2O emission is promoted by:
fert application
soil wetting
soil warming
What produces nitrate, and what removes nitrate?
produces:
nitrification
fertilizer
removes:
immobilization
dentrification
plant uptake
What causes nitrate leaching? Negative effects of leaching?
precipitation and irrigation exceeds soil water holding capacity and evapotranspiration, causing downwards water movement
negative effects of leaching:
pollution, eutrophication
start at Feb 14
nitrogen volatization
the loss of N through the conversion of ammonium to ammonia gas, which is release to the atmosphere
At pH > 9.3 [NH3] > [NH4+]
At pH <9.3 [NH3] < [NH4+]
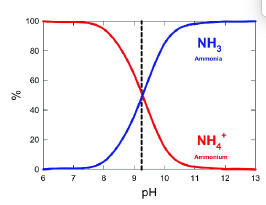
factors affecting volatization
soil pH
soil buffering capacity
water content
temperature
anhydrous NH3
82% N
basc buildinf block of most chemically derived N ferilizer
haber-bosch using N2 from air and H2 from natural gas
most is used to manufacture other N fert
easily volatilized
urea
46% N
most widely used source N
when applied to soil, urea is hydrolyzed by the enzyme urease to NH4+
subject to volatilization in high pH soils
aqua NH3
20-24% N
bulky, limited use
ammonium nitrate
33-24% N
highly explosive
Beirut explosion
ammonium sulfate
21% N
decreases soil pH more than other sources
ammonium phosphates
more often used as a P source
ammonium chloride
25% N
acid forming
important for rice
only for Cl- tolerant crops
NO30 salts
immediately available
easily leached
which of these ferts raises and which lowers pH?
urea hydrolysis and ammonifcation raise pH
nitrification decreases pH
pH changes due to N fertilizer can impact:
availability of other nutriens
NH3 volatization
solubility of SOM
slow release N fert
ex. ESN, polymer-coated ureafert
reduce N2O emissions
coated in a semi-permeable polymer or sulfur OR altered chemical formulation to decrease solubility
synchonization with plant N uptake
nutrient requirements lower in early plant stages
sufficient N required 2-3 weeks after emergence
nitrificatio and urease inhibitors
Prevent or delay hydrolysis of urea by inhibiting urease
• Inhibit nitrifying bacteria (ex: Nitrosomonas), delaying NH4
+ NO3
-
manure, compost, biosolids, plant resides
slow release over time
provides N and other nutrients (50% N, 20%, nearly all required K)
improve soil health
hetergenous
can be high salt
losses from NH3 volatilization and NO3- leaching
start at february 24
Alberta’s agricultural operation practices Act (AOPA)
to ensure the salts in manure do not affect plant growth:
manure may not be applied at rates that would result in a one ds/m increase in EC in the top 15 cm of soil
manure application is prohibited if the EC of the soil in the top 15 cm is greater than 4 dS/m
AOPA setbacks from common water for manure application on laand
4% slope or less: 30 m
4-6% slope: 60 m
6-12% slope 90 m
min setback distances for manure application
150m away from residence
30 m away from water well
10.m away from common body of water if subsurface injection is used
30 m away from a common body of water if surface applied
incorporated within 48 hours of application
under AOPA, soil NO3- N limits have been set for the top 60cm of soil. The max allowable level depends on:
productive potential
soil texture
depth to water table
soil type
fertilizer nitrogen use efficiency (NUE)
amount of N fert taken up by the crop compared to how much N fert is applied
there is a lack of spatial and temporal synchonization between N supply and crop N demand
waterlogged soils promote N loss
goals of nutrient management
maintenance of SOM
ensure plant N requirements are met
minimize environmentally damaging N losses from the soil plant system
4R nutrient stewardship
right source
select the correct source of nuttrient
balance supply of essential plant nutrients
right rate
consider the availability of nutrients from all sources
perform annual soil testing
apply nutrients to meet requirements, accounting for nutrients already in the soil
right time
avoid application when losses could be high
plan on an annual basis
right place
setback distances for applications near waterways
place nutrients where they can be taken up by growing roots
Right source
fert type
conventional, EEF, organic amedments
recognize synergism or antagonism among elements
N and P = synergistic growth
P fert can reduce Zn availability
right rate
law of diminishing returns
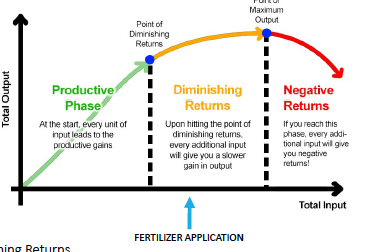
soil test
soils are highly variable
timing is critical
send to lab for: available N, P, K, Mg
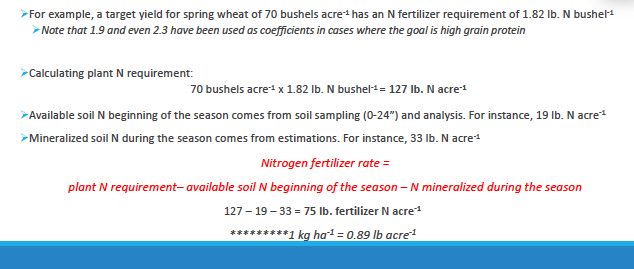
calculating N fert requirements
Nirtrogen fertilizer rate = plant N requirement- availalble soil N beginning of the season - N mineralized during the season
spring vs fall N application
spring application supplies N closer to when the crop needs it
winter and early spring leaching in colder climates
less N2O emissions in spring
Right Place
address root-soil dynamics
nutrient movement
spatial variability within the field
kinds of application
broadcast
foliar
localized
banded
seed placed