Nitrogenous compounds + polyesters module 6
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
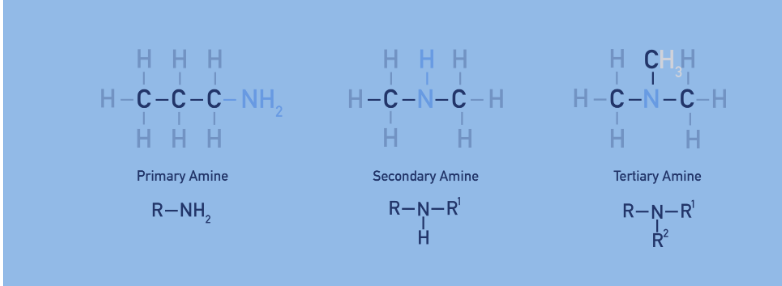
amine structures ( Image)
N attached to 1 R group = primary
N attached to 2 R group = secondary
N attached to 3 R group = tertiary
what are type of molecule are amines
Bases
lone pair on N accepts protons
How to name amines
Suffix - amine
prefix - Alkyl group (alphabetical order )
amine + acid →
Amine + Acid → Salt
(Amine + Acid ) explanation
NH₂ acts as base and accepts proton
Acid ion ionically bonds to N⁺H₃
Phenylamine + Nitric acid ( Image)
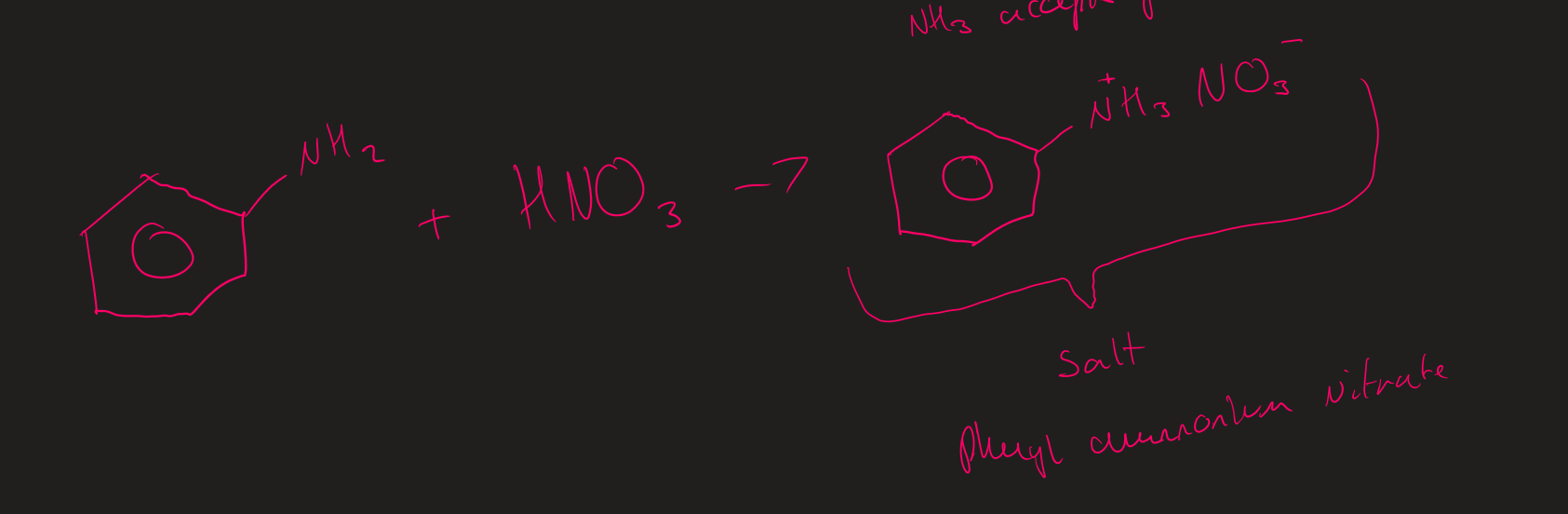
Amine + Acid how to name salt
Alkyl ammonium ion
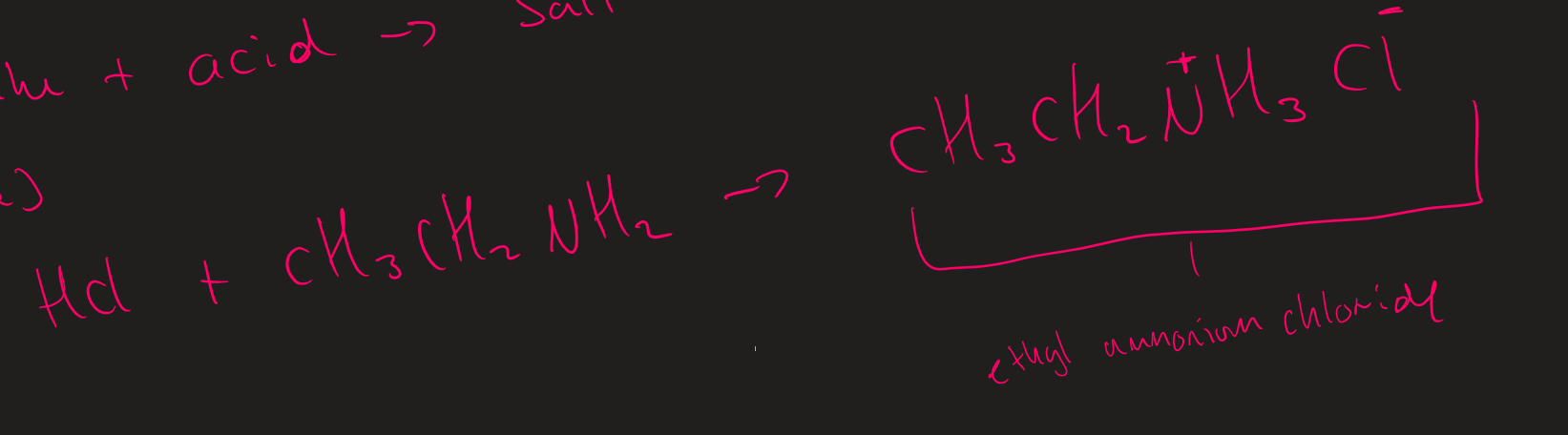
How to make an aliphatic amine reaction
Haloalkane + Excess NH₃ dissolved in ethanol → Primary amine + ammonium halide
What do amines act as in nucleophilic substitution reactions
Nucleophile → electron pair donor
Why do we use excess NH₃
Prevents further substitutions
no undesired secondary / tertiary amines
How to make a aromatic amine equation
Reduce nitrobenzene → Phenylamine
Reactants to make primary amine
Haloalkane
excess NH₃ dissolved in ethanol
By product of making a primary amine
Ammonium halide
Why is NH₃ dissolved in ethanol
Prevents H₂O from reacting with haloalkane → produces alcohol
Conditions to form a phenylamine
Sn/ HCl
reflux
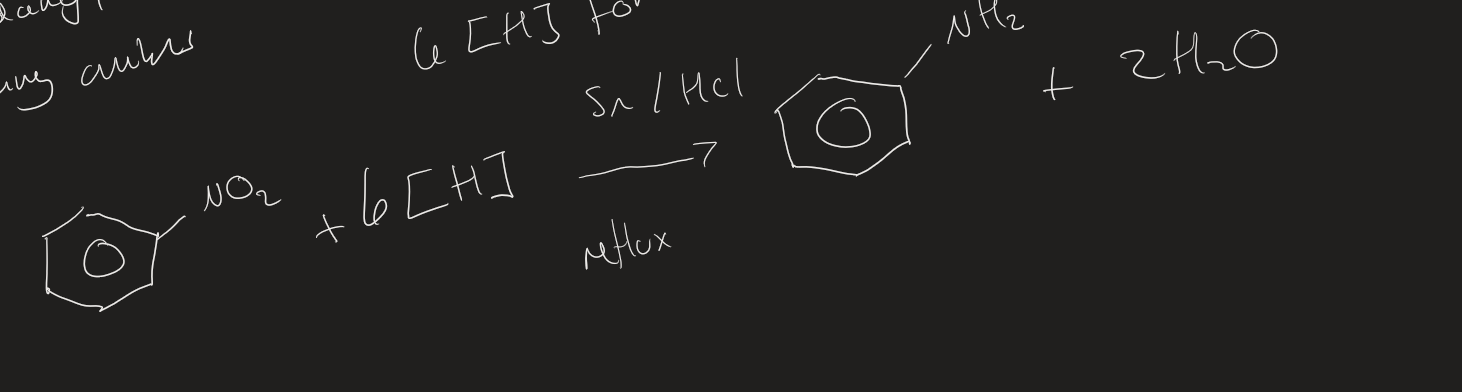
How many [ H ] to reduce 1 nitro group on nitrobenzene to form phenylamine
6 [ H ]
forming a aromatic amine word equation
C₆H₅NO₂ + 6 [ H ] → C₆H₅NH₂ + 2H₂O
forming aromatic amine by product
2 H₂O
Forming aromatic amine ( Image)
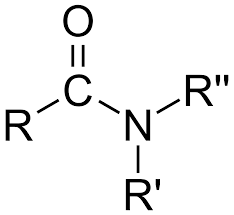
Amide structure ( Image)
CON bond
Amide structures ( Image)
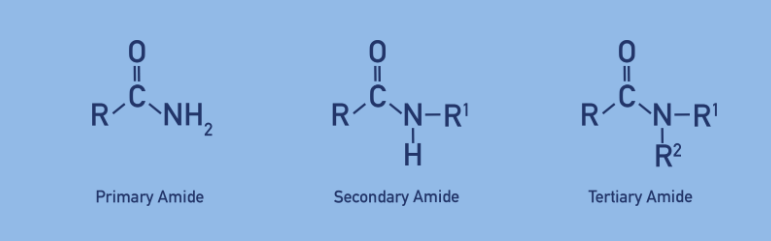
How to name primary amides
look at alkyl group then add amide
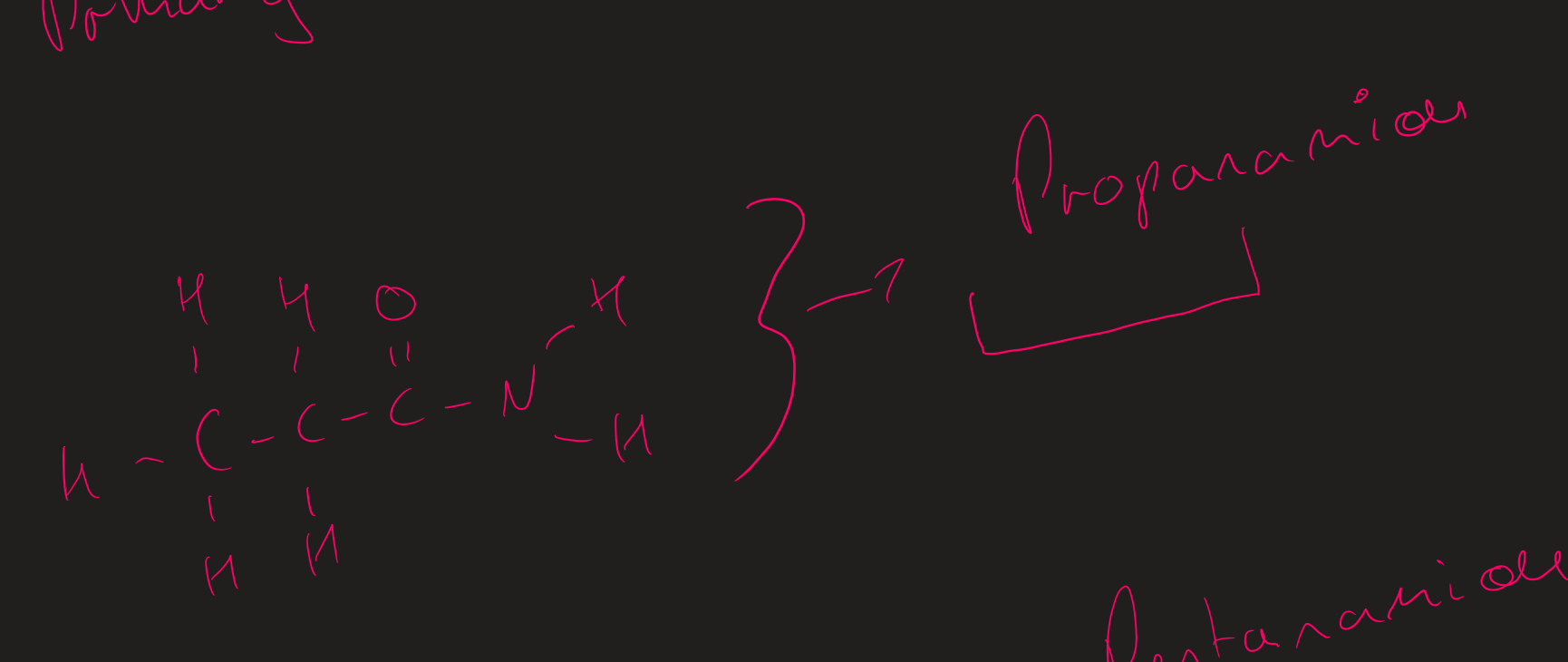
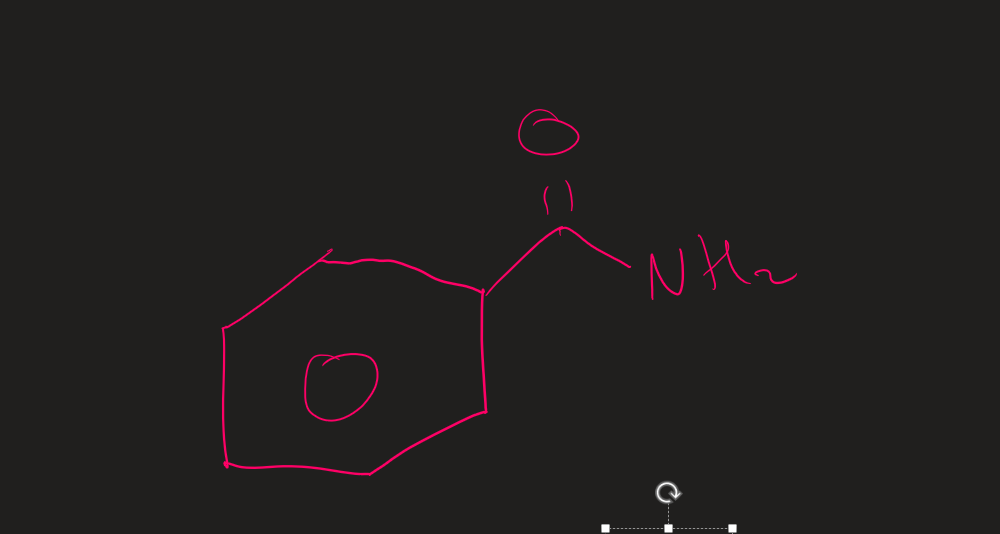
Name this structure
Benzamide
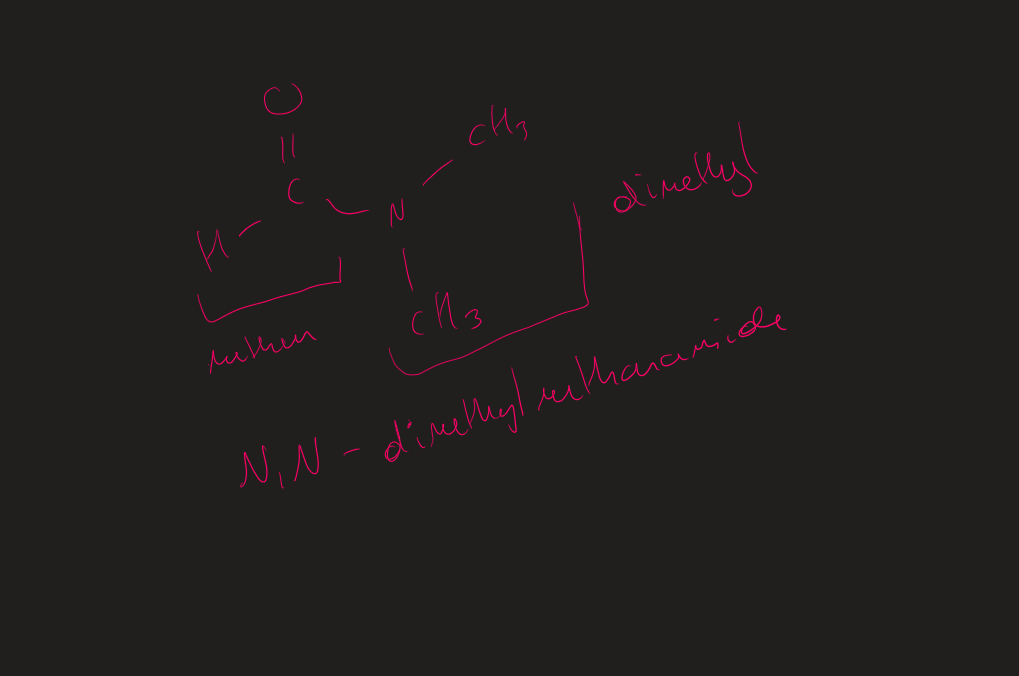
How to name secondary amines
Add N then Group attached to carbonyl = Stem + amide
R Group directly attached to N → Prefix
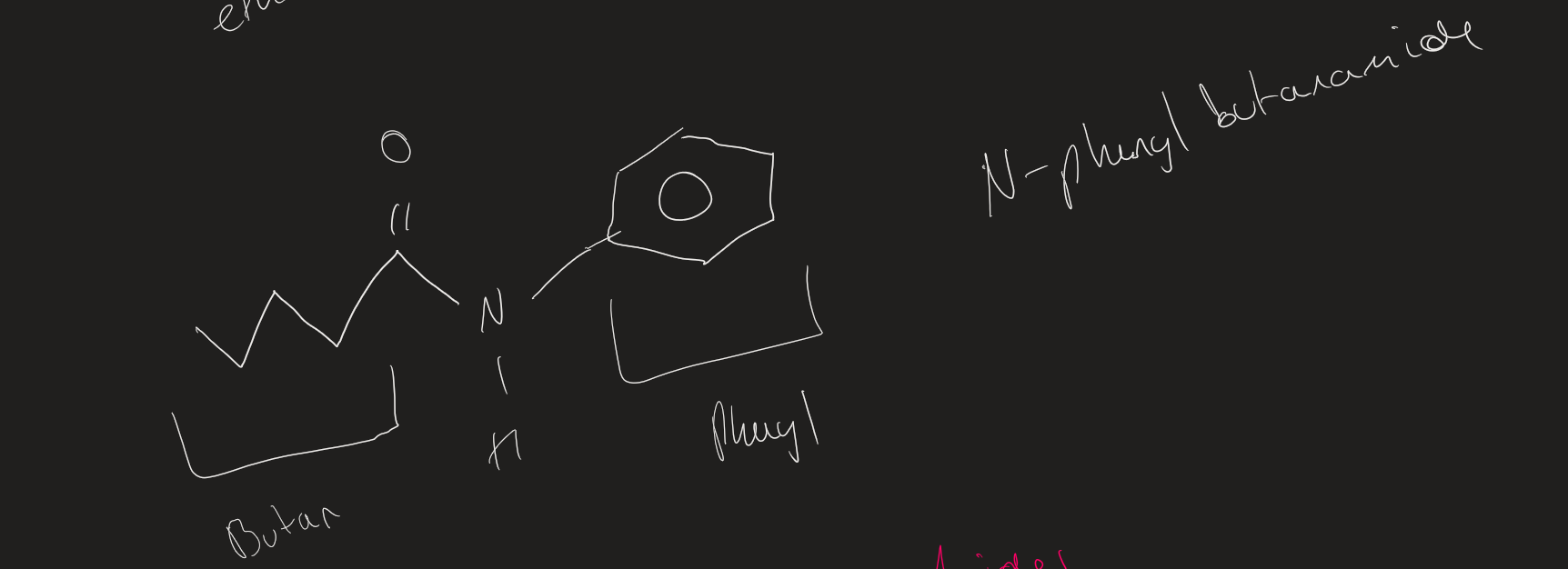
How to name tertiary amides
N,N then R group attached to N → prefix ( alphabetical order)
R attached to carbonyl = stem + amide
Primary to secondary amine mechanism
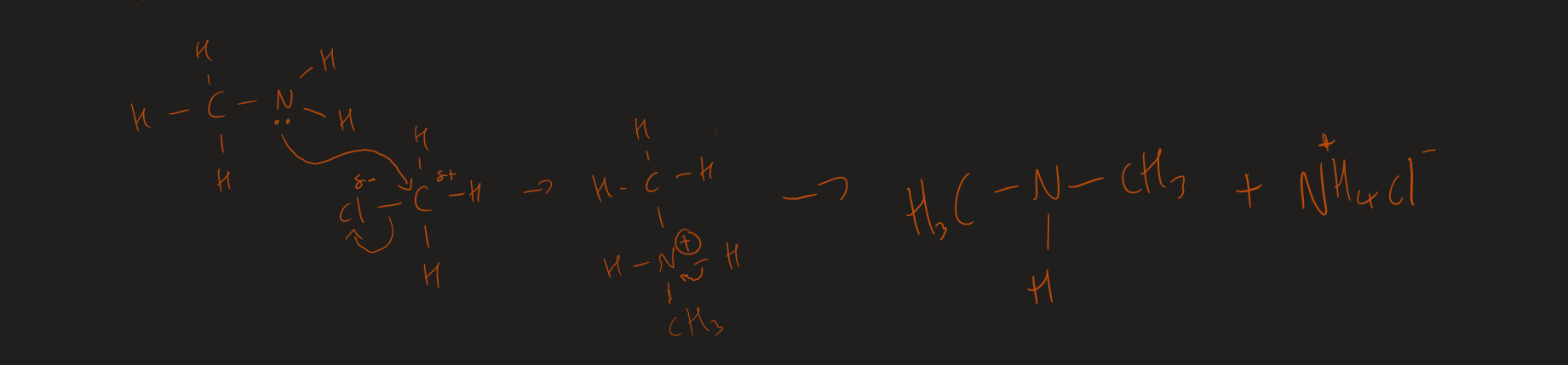
what are the ways to make a Amine
Haloalkane → Nitrile → Amine
Haloalkane + Excess NH₃ dissolved in ethanol
Acyl chloride + NH₃
Advantages of Nitrile to amine
makes only 1 product ( amine)
don’t have to purify mixture of products
How to form amine from Haloalkane ( Nitrile method)
React with KCN ( dissolved in ethanol) to form a nitrile ( 1 more carbon)
react nitrile with 2H₂ → Amine
Conditions for nitrile to amine
React with 2H₂
Nickel catalyst
ways to make a amide
Acyl chloride / acid anhydride + amine → secondary amide
what are optical isomers
Form non- imposable mirror images
Reagants and conditions for Nitrile to carboxylic acid
H₂O / HCl
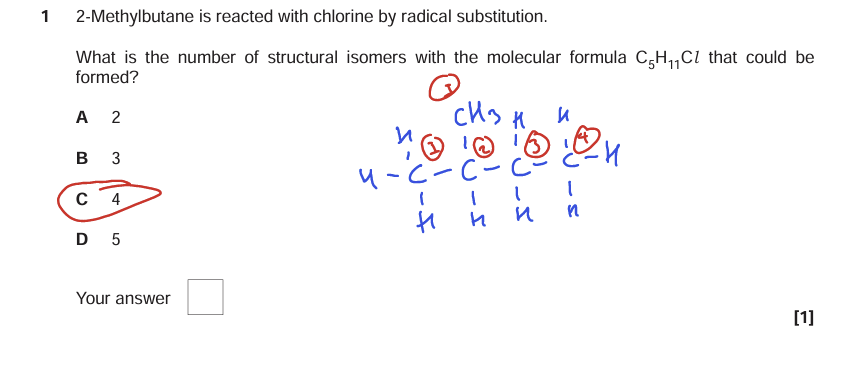
Number of structural isomers ( Image)