NMAT CHEM
5.0(1)
Card Sorting
1/156
Last updated 1:59 AM on 1/24/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
157 Terms
1
New cards
1 calorie
4.184 joules
2
New cards
No of elements
112
3
New cards
Most abundant element in the earth's crust
oxygen
4
New cards
Dalton's Atomic Theory
\>all matter is made up of tiny, indivisible atoms
\>all atoms of an element are identical but different from another element
\>atoms combine in a certain whole-number proportions ; form compounds
\>in a chemical reaction, atoms are rearranged to form new compounds; not created nor destroyed
\>all atoms of an element are identical but different from another element
\>atoms combine in a certain whole-number proportions ; form compounds
\>in a chemical reaction, atoms are rearranged to form new compounds; not created nor destroyed
5
New cards
3 Laws of Matter
\>Law of conservation of mass
\>Law of definite proportions
H2O will always be 16/2 or 8/1 kahit sa ref or kanal pa yan galing
\>Law of multiple proportions
\>Law of definite proportions
H2O will always be 16/2 or 8/1 kahit sa ref or kanal pa yan galing
\>Law of multiple proportions
6
New cards
Democritus
matter consists of invisible particles called atoms and a void (empty space). He stated that atoms are indestructible and unchangeable.
7
New cards
Dalton's model
the atom is the smallest fundamental particle of an element
8
New cards
Thomson's model
\>electrons
\>plum-pudding model
\>plum-pudding model
9
New cards
Rutherford's Model
\>atom has a positively-charged nucleus
\>gold-foil experiment (nagbombard ng alpha particles tapos biglang may nagbounce back)
\>gold-foil experiment (nagbombard ng alpha particles tapos biglang may nagbounce back)
10
New cards
Bohr's Model
\> planetary model, and energy levels that are quantized
11
New cards
Goldstein model
\>protons
\>cathode-ray tube
\>cathode-ray tube
12
New cards
JJ Thomson model
\>nature of protons and mass
13
New cards
Faraday and Davy Model
electrical nature of matter;ions
14
New cards
Chadwick model
\>neutron
15
New cards
Equation to get max no of electrons per energy level
2n^2
16
New cards
Afbau Principle
Orbitals with lowest energy level are filled first
17
New cards
Hund's rule
Each orbital receives one electron before pairing with another
18
New cards
Pauli's Exclusion Principle
No two electrons can have the same set of quantum numbers
19
New cards
Quantum numbers
\>Principal: distance of orbital from nucleus
\>Azimuthal: shape of orbital
\>Magnetic: spatial orientation
\>Spin: direction of electron spin- 1/2 up; -1/2 down
\>Azimuthal: shape of orbital
\>Magnetic: spatial orientation
\>Spin: direction of electron spin- 1/2 up; -1/2 down
20
New cards
Isoelectronic
atoms or ions with the same no of electrons
21
New cards
Representative elements
s p blocks
22
New cards
Transition elements
d block
23
New cards
Inner transitional elements
f block
24
New cards
Family/Group
columns
25
New cards
Period/Series
rows
26
New cards
Metalloids
Boring Silly Germs Are Ants Telling Politics
Boron ; Silicon ; Germanium ; Arsenic ; Antimony ; Tellurium ; Polonium
Boron ; Silicon ; Germanium ; Arsenic ; Antimony ; Tellurium ; Polonium
27
New cards
Group 1A: Alkali Earth Metals
H Li Na K Rb Cs Fr
28
New cards
Group 2A: Alkaline Earth Metals
Be Mg Ca Sr Ba Ra
29
New cards
Group 3A: Earth metals
B Al Ga In Tl
30
New cards
Group 4A: Tetrels
C Si Ge Sn Pb
31
New cards
Group 5A: Pnictogens
Nicole, Please Ask Sbarro, Bitch
32
New cards
Group 6A: Chalcogens
O S Se Te Po
33
New cards
Group 7: Halogens
Fck Chlorine Br I At
34
New cards
Group 8: Noble gases
He Ne Ar Kr Xe Rn
35
New cards
How to get formal charge
Valence electrons-Nonbonding valence electrons- Bonding electrons/2
https://i.ytimg.com/vi/BTEycFw2EkU/maxresdefault.jpg
https://i.ytimg.com/vi/BTEycFw2EkU/maxresdefault.jpg
36
New cards
Exceptions to the Octet Rule
H: 1
He, Li, Be: 2
Al, B: 6
He, Li, Be: 2
Al, B: 6
37
New cards
Molecular Geometries and Hybridization
\> (2) Linear [sp]
\> (3)Trigonal Planar; Bent [sp2]
\> (4)Tetrahedral;Trigonal Pyramidal [sp3]
\> (5)Trigonal Bipyramidal;Seesaw or Distorted Tetrahedron [sp3d]
\> (6)Octahedral; Square Pyramidal; Square Planar [sp3d2]
\> (3)Trigonal Planar; Bent [sp2]
\> (4)Tetrahedral;Trigonal Pyramidal [sp3]
\> (5)Trigonal Bipyramidal;Seesaw or Distorted Tetrahedron [sp3d]
\> (6)Octahedral; Square Pyramidal; Square Planar [sp3d2]
38
New cards
Types of Intermolecular Forces
Dipole-Dipole
Ion-dipole
London Dispersion
Hydrogen Bond
Ion-dipole
London Dispersion
Hydrogen Bond
39
New cards
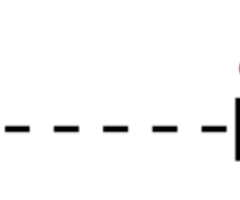
Dipole-Dipole forces
forces between polar molecules
40
New cards
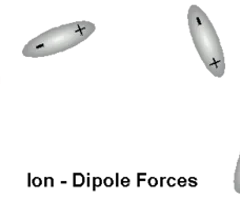
Ion-Dipole Forces
ion and polar molecule
41
New cards
London Dispersion Forces
forces between nonpolar molecules
42
New cards
Hydrogen Bond
Dipole-dipole interaction of hydrogen and F, O, N
43
New cards
Surface Tension
amount of energy required to increase the surface area of a liquid
44
New cards
Cohesion
The intermolecular attraction between like molecules
ex: water-water
ex: water-water
45
New cards
Adhesion
The force of attraction between unlike molecules, or the attraction between the surfaces of contacting bodies.
ex: water-container
ex: water-container
46
New cards
Viscosity
A liquid's resistance to flowing
47
New cards
Copper (Cu)
(I) and (II)
48
New cards
Gold (Au)
(I) and (III)
49
New cards
Cadmium (Cd)(I) and (II)
(I) and (II)
50
New cards
Lead (Pb)
(II) and (IV)
51
New cards
Antimony (Sb
(III) and (V)
52
New cards
Bismuth (Bi)
(III) and (V)
53
New cards
Mercury (Hg)
(I) and (II)
54
New cards
Iron (Fe)
(II) and (III)
55
New cards
Chromium (Cr)
(II) and (III)
56
New cards
Manganese (Mn)
(II), (III), and (IV)
57
New cards
Cobalt (Co)
(II) and (III)
58
New cards
Nickel (Ni)
(II) and (III)
59
New cards
Tin (Sn)
(II) and (IV)
60
New cards
Ammonium
NH⁴⁺
61
New cards
Sulfate
SO₄²⁻
62
New cards
Sulfite
HSO₄⁻
63
New cards
Nitrate
NO₃⁻
64
New cards
Nitrite
NO₂⁻
65
New cards
Phosphate
PO₄³⁻
66
New cards
Hydrogen Phosphate
HPO₄²⁻
67
New cards
Dihydrogen Phosphate
H₂PO₄⁻
68
New cards
Phosphite
PO₃³⁻
69
New cards
Hydroxide
OH⁻
70
New cards
Peroxide
O₂²⁻
71
New cards
Acetate
C₂H₃O₂⁻
72
New cards
Perchlorate
ClO₄⁻
73
New cards
Chlorate
ClO₃⁻
74
New cards
Chlorite
ClO₂⁻
75
New cards
Hypochlorite
ClO⁻
76
New cards
Chromate
CrO₄²⁻
77
New cards
Dichromate
Cr₂O₇²⁻
78
New cards
Permanganate
MnO₄⁻
79
New cards
Cyanide
CN⁻
80
New cards
Cyanate
CNO⁻
81
New cards
Thiocyanate
SCN⁻
82
New cards
Carbonate
CO₃²⁻
83
New cards
Hydrogen Carbonate
HCO³⁻
84
New cards
Oxalate
C₂O₄²⁻
85
New cards
Thiosulfate
S₂O₃²⁻
86
New cards
Mercury (I)
Hg₂²⁺
87
New cards
Hydronium
H₃O⁺
88
New cards
Ammonia
NH₃
89
New cards
Group IA Oxidation No.
+1
90
New cards
Group IIA Oxidation No
+2
91
New cards
Group IIIA Oxidation No
+3
92
New cards
Group IVA
+4/-4
93
New cards
Group VA Oxidation No
-3
94
New cards
Group VIA Oxidation No
-2
95
New cards
Group VIIA Oxidation No
-1
96
New cards
Noble Gases Oxidation No
0
97
New cards
Avogadro's No
6.022 x 10²³ particles
98
New cards
Activity Series
Please Stop Calling Ma A Carbon Zebra, I TIN Like Her Cute Silly Goat
Potassium Sodium Calcium Magnesium Aluminum Carbon Zinc Iron Tin Lead Hydrogen Copper Silver Gold
Potassium Sodium Calcium Magnesium Aluminum Carbon Zinc Iron Tin Lead Hydrogen Copper Silver Gold
99
New cards
Gases conversion
1 atm\=760 mmHg\=101.3 kPA
100
New cards
Boyle's Law
PV