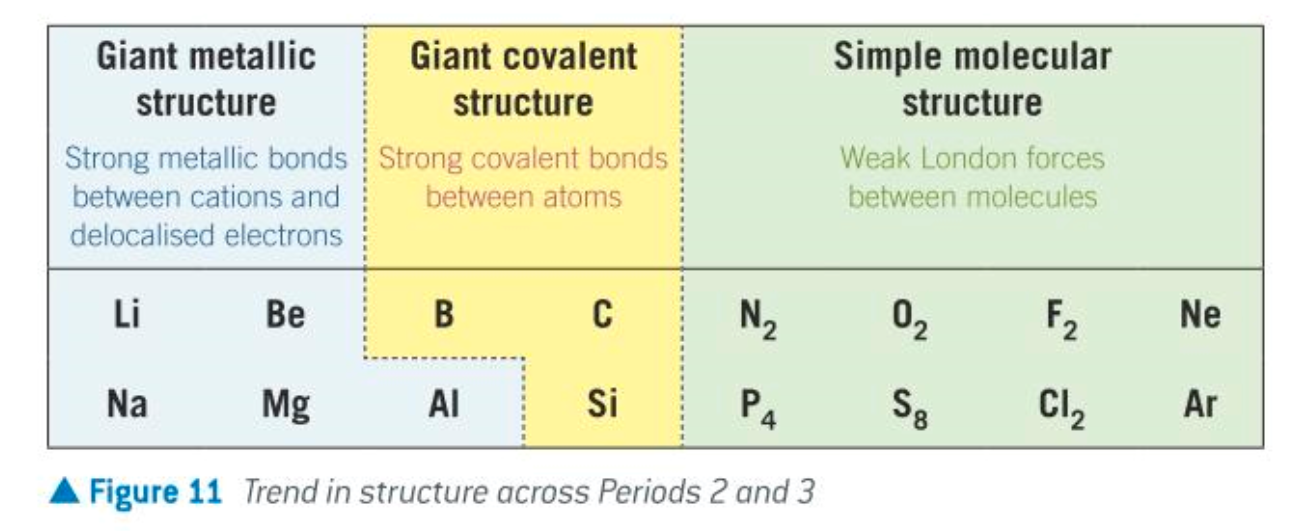
7 Periodicity
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
what do groups show and how does this affect their chemical properties
same number of e- in outer shell
same number of e- in sub shells
similar chemical properties
what do periods show
number of electron shells
periodicity def
the trends seen within groups and periods or a ‘repeating periodic pattern’
e.g. electron configuration, ionisation energy, structure, mpts
what factors affect atomic radii
atomic number
distance between nucleus and outer electrons
number of shells
how does atomic radius change across periods and groups
atomic radius decreases across a period
atomic radius increases down a group
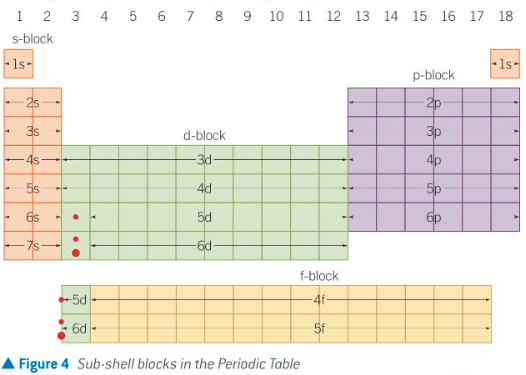
what do blocks (s-block, p-block) show on a period table
the highest energy subshell that is filled
what happens to atomic radius across a period and why
atomic radius decreases across a period
nuclear charge increases while outer electrons experience the same shielding
greater nuclear attraction on the outer shell electrons
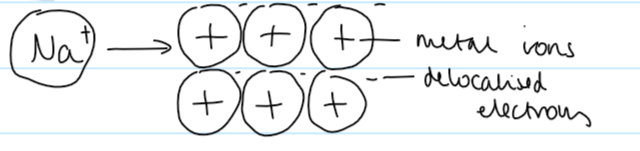
metallic bonding
strong electrostatic attraction between positive metal cations and a sea of delocalised electrons
metallic bonding diagram
what properties are looked at in this chapter 😭😭
electron configuration
ionisation energy
structure
melting points
trend of electron configuration across a period
each period starts w an electron in a new highest energy shell
across period 2, the 2s sub-shell fills w 2e-, followed by the 2p sub-shell w 6e-
across period 3, same pattern is repeated
across period 4 the highest shell number is n = 4, from the n = 4 shell only the 4s and 4p sub-shells r occupied as the 3d sub-shell is involved
trend of electron configuration down a grp
elements in each group have atoms w the same no. e- in their outer shell
elements in each grp also have atoms w the same no. e- in each sub0shell
this gives elements in the same grp similar chemistry
how are elements divided into blocks
corresponding to their highest energy sub-shell
blocks on periodic table pic
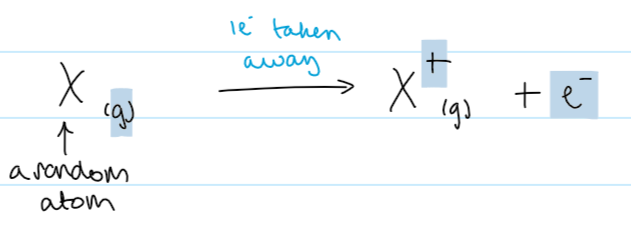
first ionisation energy def
energy required to remove 1 mole of electrons from 1 mole of gaseous atoms of an element to form 1 mole of 1+ ions
factors that affect 1st ionisation energy
atomic radius
electron shielding
nuclear charge
(mention all in exam Qs)
how does atomic radius affect IE
if larger atomic radius
outer electron is further from nucleus
e- has less nuclear attraction
less energy needed to remove
easier to remove outer e-
how does e- shielding affect IE
shielding effect means more e-
therefore more repulsion
outer e- has less nuclear attraction
less energy required to remove it
easier to remove outer e-
how does nuclear charge affect IE
more protons → higher nuclear charge
outer e- has a greater nuclear attraction
more energy required to remove the outer e-
harder to remove outer e-
1st IE equation (using X as a random element)
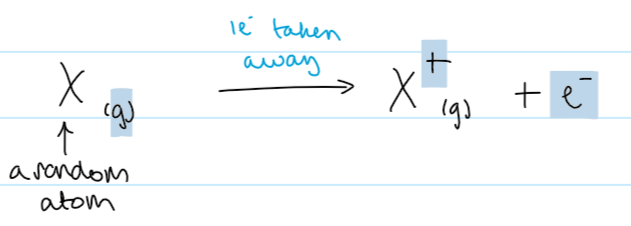
2nd IE def
energy required to remove 1 e- from each ion in 1 mole of gaseous 1+ ions of an element to form 1 mole of gaseous 2+ ions
why does Al have a higher bpt than Na
Al 3+ ions have a greater charge than Na+ ions
therefore more delocalised electrons
stronger electrostatic attractions between cation and delocalised electrons
more energy needed to overcome
higher bpt
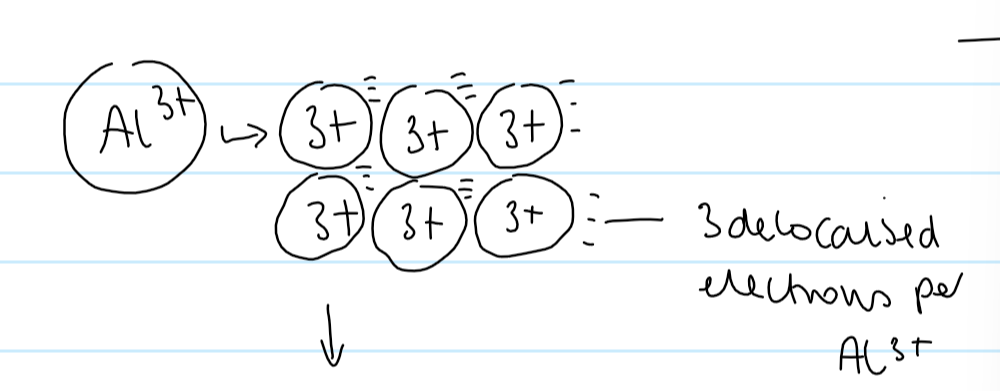
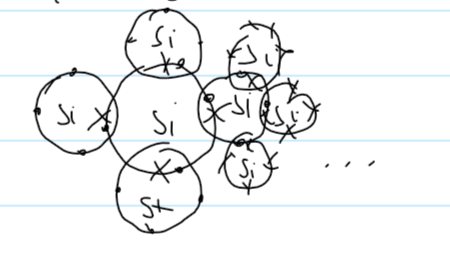
structure of Si
giant covalent structure
breaking strong covalent bonds
lots of energy to overcome
how many other Si is Si covalently bonded to
each Si is covalently bonded to 4 others
how does P travel
P4
how does S travel
S8
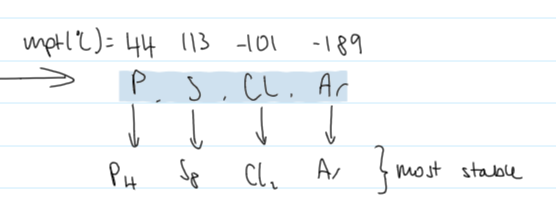
why does P and S have a higher bpt than Cl and Ar all in period 3
P4
4 atoms
more e-
stronger LF
more energy required to overcome
S8
much higher mpt&bpt expected of a simple molecular structure
8 atoms
many more e-
stronger LF
more energy required to overcome
what type of IMF r present from P4 to Ar
no polar bonds therefore no permanent dipole-dipole interactions
no HB
only LF
how does bpt change down grp17 (7) (halogens)
all simple covalent/molecular
all diatomic
all LF
down the grp atomic radius increases
more e-
stronger LF
more energy to overcome
bpt increases down grp7
bpt down grp1
bpt decreases down group
metallic lattice structure
all 1+ ions attracting 1e-
delocalised e- have more attraction higher up the grp
closer to nucleus due to smaller atomic radius
stronger electrostatic attraction
more energy to overcome (higher up the grp)
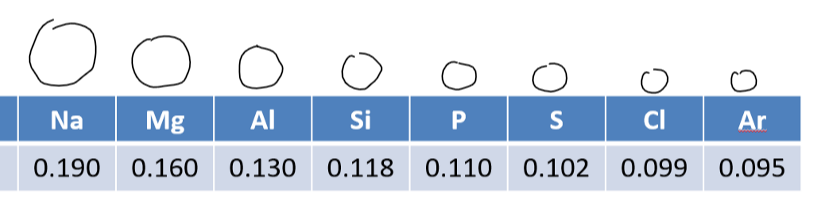
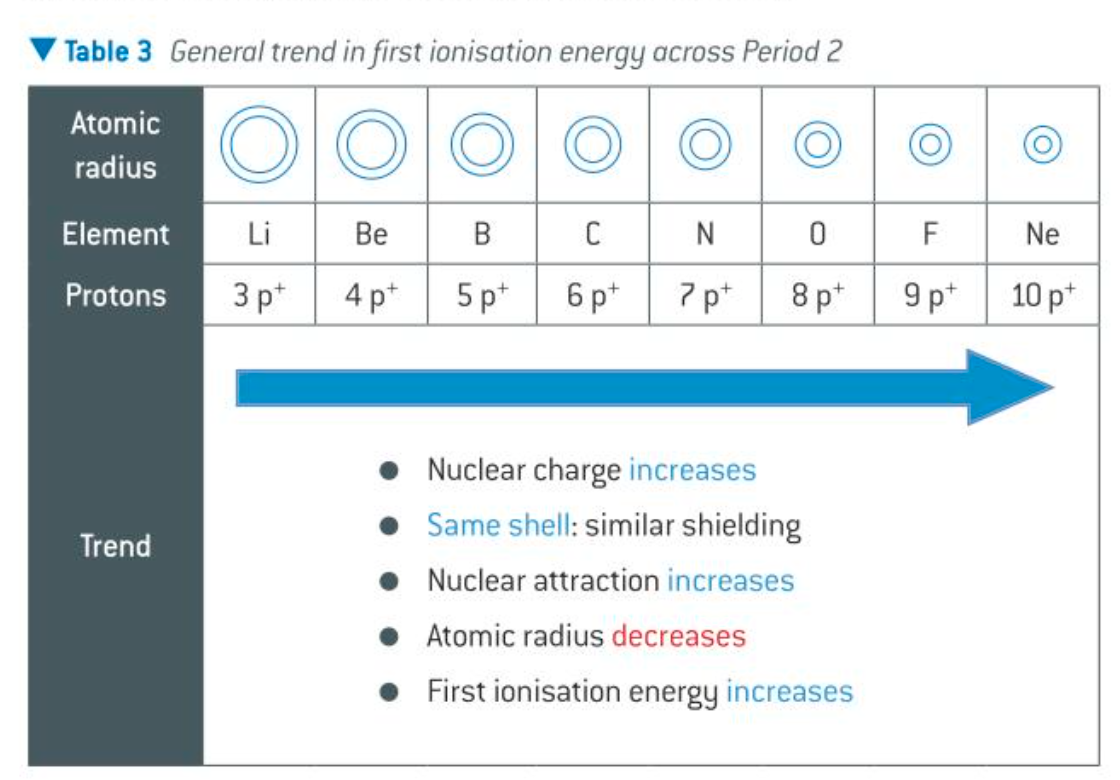
atomic radii across period
decreases across a period
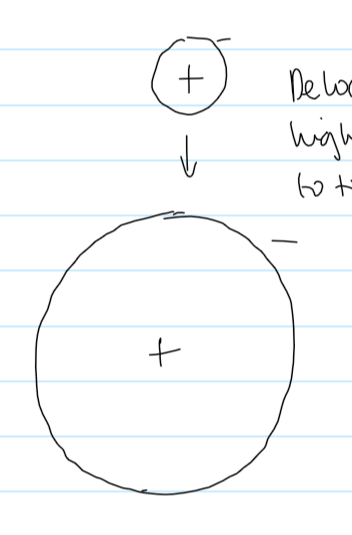
atomic radius exam q ans
atomic radius decreases across period
nuclear charge increases while outer electrons experience the same shielding
greater nuclear attraction on the outer shell electrons
1st IE down grp1 graph
decreases
easier to remove outer e- down grp1
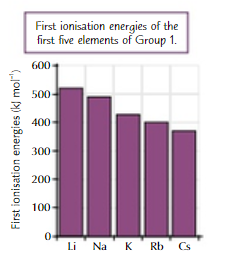
explain why 1st IE decreases down a group (1)
first IE decreases down a group where the outer e- is easier to remove
although nuclear charge increases, so does the atomic radius
outer electron further from nucleus
less nuclear attraction
more electron shielding therefore greater shielding effect therefore greater repulsion between electrons
less nuclear attraction to outer electron
less energy require to remove the outer electron
1st IE across period 2 and 3
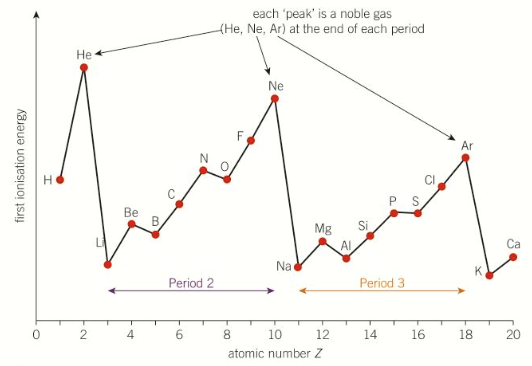
explain 1st IE trend across a period
across a period the 1st IE increases - harder to remove the outer e-
across a period nuclear charge increases - more nuclear attraction
similar electron shielding because the outer electrons are in the same energy level
atomic radius decreases across a period
overall the outer e- has more attraction to the nucleus across a period - more energy required to remove it
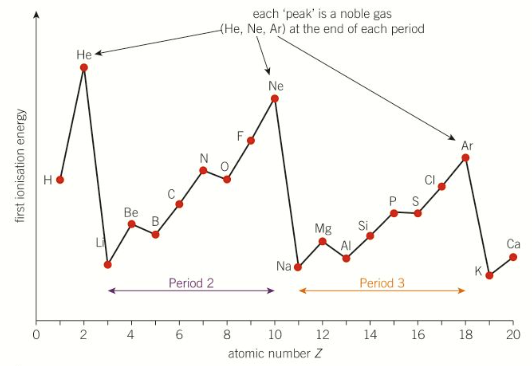
where are there drops in period 2 and why
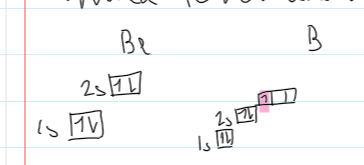
grp2&3 and group5&6, Be → B and N → O
B (grp3) outer e- is in a higher energy level than Be (grp2)
more e- shielding
less nuclear attraction
less energy required to remove despite higher nuclear charge
explain y there is a drop from N → O
for O (grp6) one p-orbital is doubly filled
repulsion between 2e- in orbital
outer e- has less nuclear attraction
despite higher charge
less energy to remove
why do successive IEs increase within a shell
bcuz u r removing an e- from an increasingly positive ion
greater nuclear attraction
electron configuration for chromium
1s2 2s2 2p6 3s2 3p6 3d5 4s1
electron configuration for copper
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰
1st IE of oxygen
O(g) -> O(g)+ + e-
describe the structure of metals
billions of metal atoms held together by metallic bonding in a giant metallic lattice
in a solid metal structure each atom has donated its negative outer shell electrons to a shared pool of electrons which are delocalised throughout the whole structure
the delocalised e- are mobile and able to move throughout the structure.
why do metals have high mpts and bpts
most metals high temperatures are needed to provide the large amount of energy needed to overcome the strong electrostatic attraction between the cations and electrons
strong attractions → high mpts and bpts
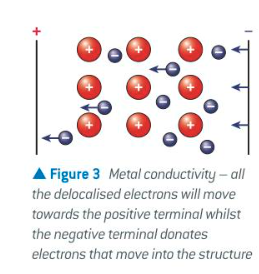
why can metals conduct electricity when solid or molten
when a voltage is applied across a metal the delocalised e- can move through the structure carrying charge
has mobile charge carriers
are metals soluble
no, any interactions w the charges in a metallic lattice would lead to a reaction rather than dissolving
why do giant covalent structures have high mpts and bpts
has strong covalent bonds
high temperatures needed to provide the large amount of energy needed to break the strong covalent bonds
would diamond be soluble in water and why
no
giant covalent lattices are insoluble in almost all solvents
the covalent bonds holding together the atoms in the lattice are too strong to be broken by interactions w solvents
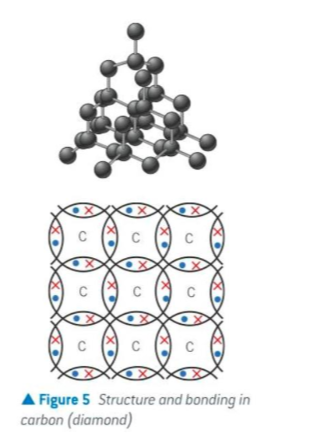
why can’t diamond conduct electricity
all 4 outer shell e- are involved in covalent bonding
none available for conducting electricity
what would a dot and cross diagram of structure and bonding in carbon (diamond) look like
how many carbons is each carbon bonded to in graphite, what is the bond angle ?
3, 120°
how can graphite conduct electricity if its a non-metal
the bonding in the hexagonal layers only uses 3 of carbon’s 4 outer shell e-
the spare e- is delocalised between layers
therefore there is a mobile charge carrier
can conduct electricity
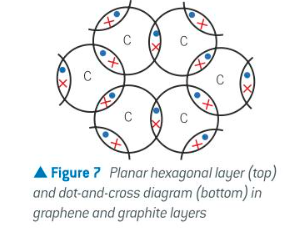
what is graphene
a single layer of graphite
can graphene conduct electricity
yes, there is 1 delocalised e- that’s free to move
structure of graphite
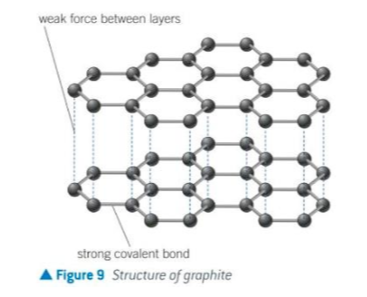
what is between the hexagonal layers of graphite
weak forces
dot and cross of a graphene layer
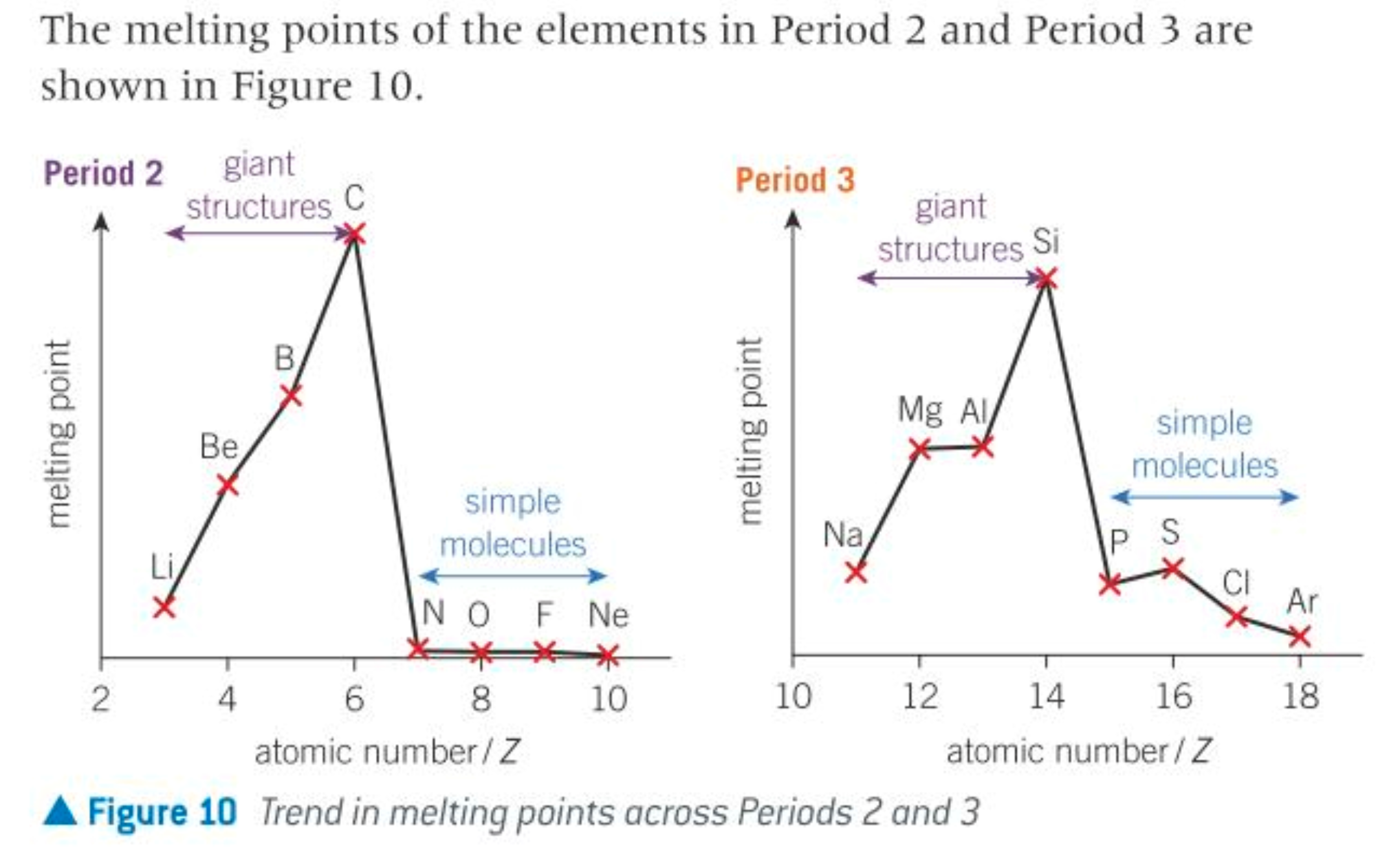
why is there a decrease in mpt from group4-5
the sharp decrease in mpt is due to the change from a giant covalent structure to simple molecular structure
giant covalent structures have strong covalent bonds between atoms, higher temps needed to provide the energy to break them
simple molecular structures only have weak LF between molecules that don’t take much energy to separate