water of crystallisation
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
hydrated ions
ions which are surrounded by water molecules
ion dipole forces
forces holding these hydrated ions together
Water of crystallisation refers to...
water molecules woven into a salt’s solid crystal lattice
hydrated salt
If a salt contains water of crystallisation
anhydrous salt
If a salt doesn’t contain water of crystallisation
How would we represent this hydrated salt?
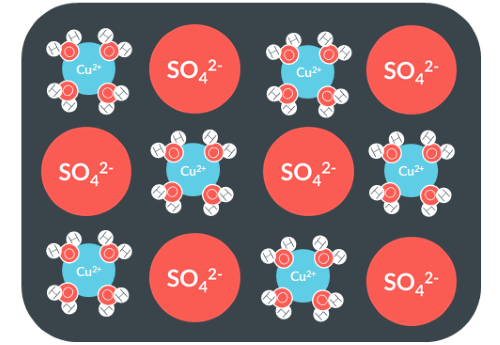
CuSO4.4H2O
How would we represent this hydrated salt?
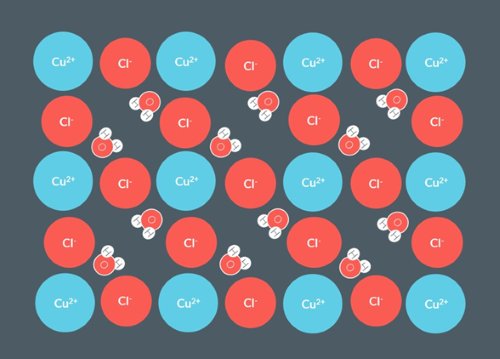
CuCl2.2H2O
We can turn a hydrated salt into an anhydrous salt by...
heating it vigorously
It’s virtually impossible to turn a sample of an anhydrous salt into a hydrated salt in one step because…
it requires adding an exact amount of water
a small amount of the anhydrous salt will dissolve in the water
we can’t ensure that the water added will be distributed evenly throughout the sample
Anhydrous copper sulfate can be added to a sample of liquid to test for water.
If the copper sulfate dissolves in the liquid to form a blue solution or turns into a blue solid, this means…
there is water in the sample
Anhydrous copper sulfate can be added to a sample of liquid to test for water.
If the copper sulfate remains as a white powder, this means…
there isn’t water in the sample
Anhydrous salts are mainly used…
to test for water
to remove water from a reaction mixture
to remove water from a solvent