polymers and giant structures (covalent bonding)
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
what are polymers and giant covalent structures?
large covalent molecules
what do giant ionic and covalent structures form?
huge continuous networks of atoms that are bonded together and cannot be separated into individual units without breaking bonds.
what are 2 common polymers and their uses?
polythene - plastic bags
polyvinyl chlorine (PVC) - industrial applications and water pipe production
structure of ethene and polythene:
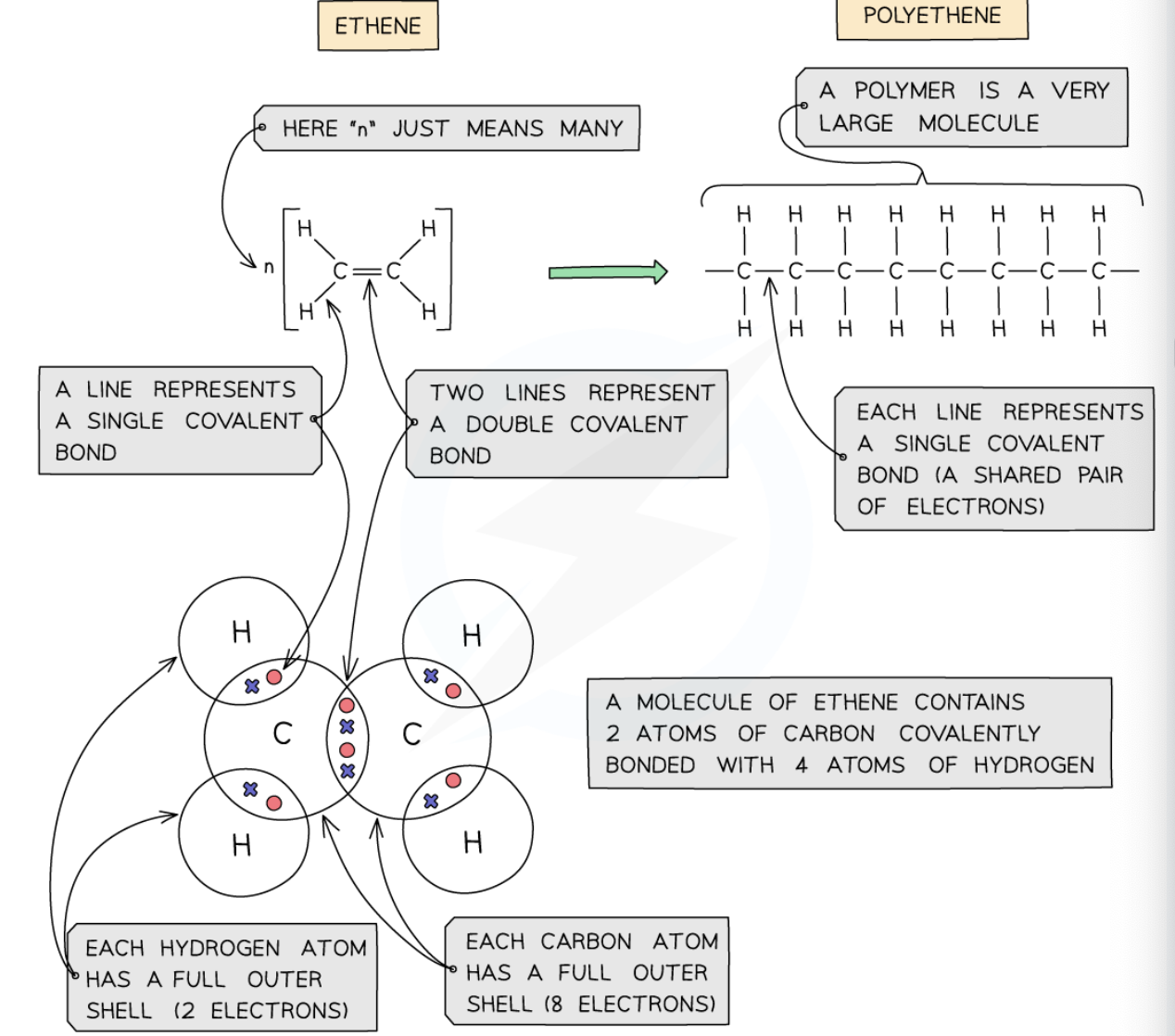
what are some giant covalently bonded substance examples?
graphite, diamond, and silicon dioxide
what do giant covalently bonded substances form?
giant crystal structures made from many atoms held together by covalent bonds
why are polymers and giant structures solid at room temperature?
the larger the molecule, the more intermolecular forces that need to be overcome, so the B.P is higher and they are solid at room temperature