chem exam 2 - unit 6 the mole
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1 mole
6.023 × 10^ 23
Avogadro’s number
unit to measure chemical quantities and number of atoms/molecules
finding molar mass
atomic mass = molar mass
ex: atomic mass of Carbon (C) = 12.01 amu → molar mass = 12.01 g/mol
moles to particles
Mole x 6.023 × 10^ 23/1 mole = particles
particles to moles
particles x 1 mole.6.023 × 10^ 23 = mole
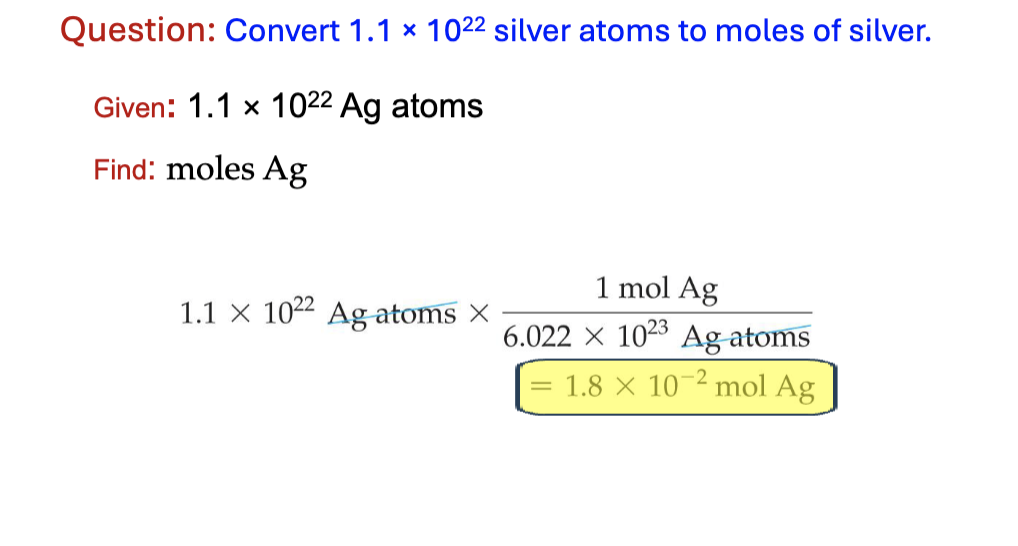
mole to mass
mole x molar mass/1 mole = mass
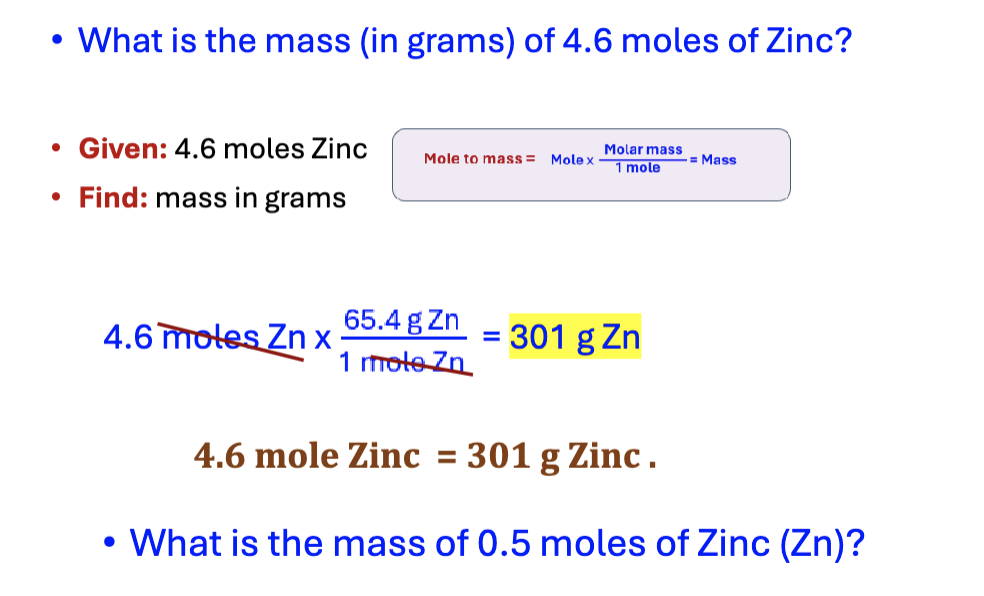
mass to mole
mass x 1 mole/molar mass = mole
volume (L) to moles OR moles to volume (L)
V to M = volume x 1mole/22.4 L = moles
OR
M to V = moles x 22.4 L/1 mole = volume
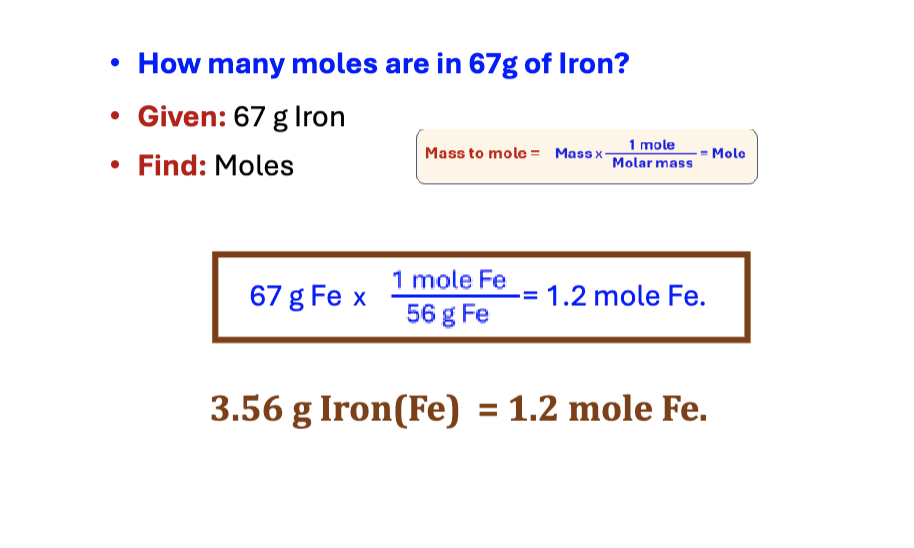
mass to particles
mass x 6.023 × 10^ 23/molar mass = atoms
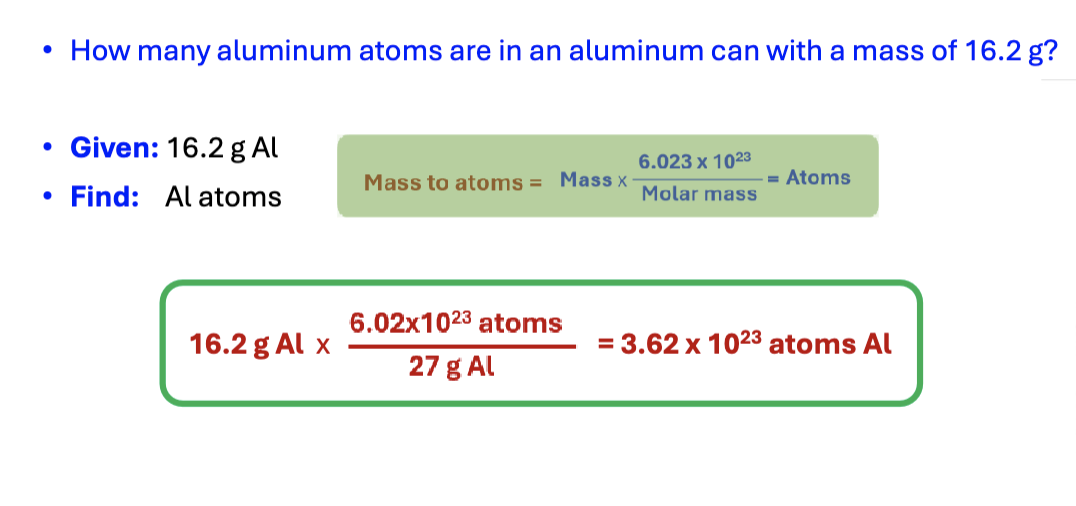
particles to mass
atoms x molar mass/6.023×10^ 23 atoma = mass
how to find molar mass of molecules
ex: C6H6
multiply the subscript values of each element by their own molar mass and add products together
6×12 + 6×1 = 78 molar mass
78 g of C6H6 = 1 mol
how to find molar mass of formula units
sum of the molar mass of the constituent atoms
ex: CaCO3:
1 Ca = 40 g
1 C = 12 g
3 O = 3(16) g = 48 g
40+12+48 = 100 g = 1 mole = formula mass of CaCO3
how to find amount of formula units in a compound
Step 1: Find the molar mass of MgCl₂
Mg = 24.305 g/mol
Cl = 35.45 g/mol × 2 = 70.90 g/mol
Molar mass of MgCl₂=24.305+70.90=95.205 g/mol
🔹 Step 2: Convert grams to moles moles of MgCl₂= 300 g/95.205 g/mol≈3.15 mol
🔹 Step 3: Convert moles to formula units
Use Avogadro’s number:
1 mol=6.022×1023 formula units
formula units of MgCl₂=3.15 mol×6.022×10^23≈1.90×10^24
✅ Final Answer: 1.90×10^24 formula units of MgCl₂
percent composition
“each element’s mass in a molecule compared to the total mass of the compound”
percent composition = mass of element/mass of molecule x 100
ex: percent comp of carbon = 12g/16g x 100 = 75%
empirical formula
rations of elements present in the compound (but NOT the actual number of atoms in the molecule)
subscripts are the smallest whole numbers that indicate the ratio of elements
ex: C20H12
empirical formula = C5H3