electrochem/redox
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
electrochemical processes
redox reactions where energy released by spontaneous reaction is converted to electricity
OR electrical energy is used to cause a non-spontaneous redox reaction to occur
disproportionation reaction
redox reaction whereby an element undergoes oxidation and reduction simultaneously
anode
electrode where oxidation occurs
cathode
electrode where reduction occurs
how is electricity generated through a spontaneous redox reaction?
oxidation reaction and reduction reaction take place simultaneously in separate locations
transfer of electrons through external metal wire
transfer of ions through salt bridge
reaction progresses, sets up constant flow of electrons, generates electricity
purpose of salt bridge
complete the electrical circuit: allowing the movement of ions between half-cells
maintain electrical neutrality: prevent build-up of positive charges at anode/build-up of negative charges at cathode → this would prevent cell from operating
types of half-cells
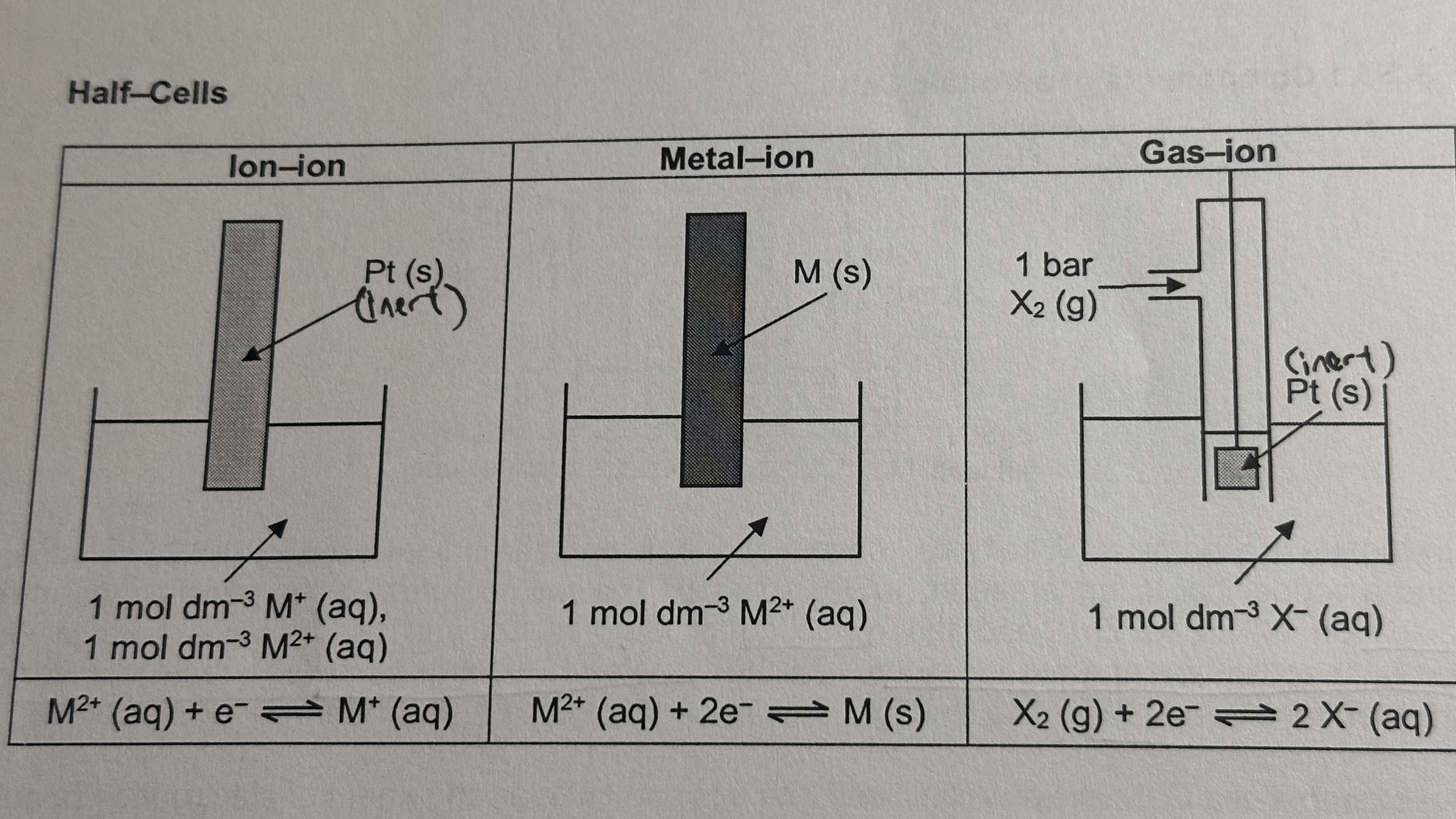
ion-ion
platinum (inert) electrode submerged in solution that is mixture of two metal ions
metal-ion
metal (reactive) electrode submerged in solution of metal ions
gas-ion
gas bubbling over platinum (inert) electrode submerged in gaseous ion solution
standard reduction potential
aka standard electrode potential
the electromotive force between a half-cell when connected to standard hydrogen electrode under standard conditions (1 mol dm-3, 298K, 1 bar)
by comparing half-cell against standard hydrogen electrode, it determines the tendency for the forward reduction reaction (see data booklet) under standard conditions
conditions for measuring standard reduction potential
298K
1 bar pressure for gases
1 mol dm-3 for ions
use platinum electrode if half-cell doesn’t involve metal
salt bridge completes circuit
factors affecting electrode potential
temperature and pressure
nature of metal
concentration of ions
medium where reaction takes place (eg acidic conditions? → see redox table)
standard hydrogen electrode
1 bar hydrogen gas bubbling over platinum electrode immersed in 1.00 mol dm-3 solution of H+ (eg HCl) under standard conditions (298K, 1 bar)
standard reduction potential = 0V because at equilibrium
IA9 Q4: what is the expected cell potential for a voltaic cell made from standard copper half-cell connected to magnesium half cell where concentration of Mg2+ ions was 0.001 mol dm-3?
Mg2+ + 2e ⇌ Mg where Eθ(Mg2+/Mg) = -2.37V (for 1.00 mol dm-3)
when concentration of Mg2+ decreases (from standard 1.00M to 0.001M), by le chatelier’s principle, POE shifts left, extent of forward reaction decreases
E(Mg2+/Mg) becomes more negative
Ecell = Eθ(Cu2+/Cu) – more negative Eθ(Mg2+/Mg) therefore Ecell increases
the more positive the Eθ value,
POE lies on right
forward reaction (reduction) favoured
oxidising agent (LHS) higher tendency to gain e-, stronger oxidising agent/itself higher tendency to be reduced
the more negative the Eθ value,
POE lies on left
backward reaction (oxidation) favoured
reducing agent (RHS) higher tendency to lose e-, stronger reducing agent/itself higher tendency to be oxidised
standard cell potential
maximum potential difference between electrodes. the tendency of electrons to flow through the external circuit of a voltaic cell under standard conditions (1 mol dm-3, 298K, 1 bar).
Eθcell = Eθcathode/reduction – Eθanode/oxidation
note: Eθcell is always positive for a battery / DO NOT CHANGE THE SIGNS OF ANY VALUES FROM DATA BOOKLET WHEN USING EQUATION
Eθcell > 0
ΔG < 0 → spontaneous forward redox reaction, non-spontaneous backward redox reaction
Eθcell < 0
ΔG > 0 → spontaneous backward redox reaction, non-spontaneous forward redox reaction
Eθcell = 0
ΔG = 0 → equilibrium. rate of forward reaction = rate of backward reaction.
uses of Eθcell
predict feasibility of reaction (based on ΔG)
however, might not be accurate if:
very high Ea needed for reaction → kinetically slow
not standard conditions
uses of Eθ
determine strength of OA/RA
predict direction of electron flow (based on above)
why is potassium manganate (VII) not a primary standard?
difficult to obtain pure because it is reduced by substances in distilled water to form maganese (IV) dioxide
presence of maganese (IV) dioxide further catalyses auto-decomposition of the potassium manganate solution: 4MnO4- + 2H2O → 4MnO2 + 3O2 + 4OH-
manganate (VII) is inherently unstable in the presence of maganese (II) ions: 2MnO4- + 3Mn2+ + H2O → 5MnO2 + 4H+
reaction is slow in acid but fast in neutral solution
sodium thiosulfate and iodine colour change
added to iodine → brown iodine fades
near endpoint, starch solution added → blue-black colour produced because starch-iodine complex formed
with continued addition of sodium thiosulfate, blue-black colour disappears because iodine is reduced by sodium thiosulfate
I2 + 2S2O3 2- → 2I- + S4O6 2-
solution of FeSO4 titrated with acidified KMnO4 colour change
Before endpoint:
All purple KMnO₄ added is immediately reduced to colourless Mn2+.
The solution turns from green (Fe²⁺) → yellow (Fe³⁺).
At the endpoint:
All Fe²⁺ is oxidized to Fe³⁺.
The first drop of KMnO₄ that remains unreacted imparts a pale pink colour to the yellow solution: observed as orange
green → yellow → orange (yellow + pink)
balanced equation for solution of FeSO4 titrated with acidified KMnO4
5Fe2+ + MnO4- + 8H+ → 5Fe3+ + Mn2+ + 4H2O
activity series
ranks metals according to the ease with which they undergo oxidation to form cations
gives information of which single replacement reactions occur spontaneously, but not how fast they occur
reactions of metals with water
metals above H in reactivity series displace H+ ions from cold water to form hydrogen gas and metal hydroxide: 2M (s) + 2H2O (l) → 2M+ (aq) + OH- (aq) + H2 (g)
metals slightly lower but still above H will displace H+ ions from steam to form hydrogen gas and metal oxide: Mg (s) + H2O (g) → MgO (s) + H2 (g)
metals below hydrogen do not undergo reaction
reactions of metals with acids
metals above H in reactivity series will displace H+ ions from dilute acid to form hydrogen gas and metal salt: Zn (s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
anions of some conc acids can act as oxidising agents for metals below H: Cu (s) + 4HNO3 (aq) → Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
displacement reactions of metals with other cations
metal: itself oxidised → reducing agent: higher in reactivity series, stronger RA since readily lose electrons
metal cations: itself reduced → oxidising agent: lower in reactivity series, stronger OA since readily gain electrons
metal (s) displaces ions of metal below it in reactivity series to form its own ions
solid displace ions in aq solution
solid displace ions in solid [thermite reaction: heat together, exothermic reaction, forms solid compound and molten metal]: Fe2O3 (s) + 2Al (s) → 2Fe (l) + Al2O3 (s)
reactions of metals
in water
much higher than H: reacts with cold water → forms hydrogen gas and aqueous metal hydroxide
slightly higher than H: reacts with steam → forms hydrogen gas and solid metal oxide
in acid
higher than H: forms hydrogen gas and salt
lower than H: oxidised by anion of HNO3 acid → forms NO2 gas, water and the aqueous ionic compound
displacement
displace aqueous ions → forms solid metal and aqueous ion
displace solid ions [thermite reaction] → forms molten metal and solid compound
electrolysis
process of passing a direct current of electricity through an electrolyte to cause a non-spontaneous chemical reaction
electrolyte
molten compounds or aqueous solutions which can conduct electricity
voltaic vs electrolytic cell
voltaic 2 redox reactions taking place simultaneously in separate locations, transfer of electrons through wire and transfer of ions through salt bridge VS electrolytic reactants are not separated
voltaic is spontaneous redox reaction producing electricity VS electrolytic is supplying electricity so that non-spontaneous redox reaction occurs
factors determining which ion is preferentially discharged at each electrode
standard electrode potentials of ions
cathode (reduction, forward reaction) → more positive preferred
anode (oxidation, backward reaction) → more negative/less positive preferred
relative concentration: only when difference in E is small!
by LCP, conc of Cl- (on RHS of eqn) increase, POE shifts left, favour backward reaction (oxidation) more → E becomes more negative/less positive → if exceeds E of H2O, Cl- higher tendency for oxidation, preferentially discharged
higher concentration, higher tendency to be reduced/oxidised
nature of electrodes (for anode only, since cathode undergoes reduction and metals undergo oxidation)
inert will not participate in reaction
reactive anode (eg Cu) will be oxidised. ECu2+/Cu is the least positvie/most negative, higher tendency for oxidation, preferentially discharged → application: purification
note: graphite inert but oxygen produced at anode will oxidise the carbon anode, forming CO2/CO which contaminates the oxygen
faraday’s 1st law
mass of any substance liberated or deposited at anode electrode during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte.
m ∝ Q
faraday’s 2nd law
no. of faradays F required to discharge one mole of an ion at an electrode is equal to the number of charges on the ion
Q = I x t = ne x F
ne is the no. of moles of electrons. use this and mole ratio to calculate no. of mol of product/reactant.
factors affecting mass of products in electrolysis
time t
magnitude of direct current I
charge on ion of element → affects mole ratio and thus ne
explain what ion mixture is in cathode
the half reaction with more positive standard electrode potential → so that the emf across cell is positive