2.1.4 Enzymes
5.0(1)
Card Sorting
1/43
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
1
New cards
what are enzymes?
biological protein catalysts that speed up reactions
2
New cards
what do enzymes do?
lower the activation energy (Eₐ) of a reaction - different reactions have different activation energies
they do this two ways:
1) to join two substrate molecules, it holds them together
2) to catalyse a break down, fitting into the active site puts strain on the bonds in the substrate and they break easier
they do this two ways:
1) to join two substrate molecules, it holds them together
2) to catalyse a break down, fitting into the active site puts strain on the bonds in the substrate and they break easier
3
New cards
what is metabolism?
all reactions that take place in the body
4
New cards
what is metabolic rate?
rate of all chemical reactions in the body
5
New cards
what two types of reactions do enzymes catalyse?
anabolic - making bonds and making the product, needs energy
catabolic - breaking bonds and breaking the substrate, releases energy
catabolic - breaking bonds and breaking the substrate, releases energy
6
New cards
what is Vmax?
the maximum rate of enzyme controlled reactions
7
New cards
what are examples of reactions catalysed by enzymes?
protein synthesis
cellular reactions
photosynthesis
DNA replication
digestion
cellular reactions
photosynthesis
DNA replication
digestion
8
New cards
what is the structure of enzymes?
large 3D globular protein molecule
shape is determined by tertiary structure
soluble in water - due to position of the hydrophilic and hydrophobic R groups
have a groove called the active site
shape is determined by tertiary structure
soluble in water - due to position of the hydrophilic and hydrophobic R groups
have a groove called the active site
9
New cards
what is collision theory?
for a reaction to take place molecules need to collide in the right orientation
high temperature and high pH increase kinetic energy and speed - this increases the number of successful collisions and the rate of reaction
activation energy needs to be supplied for a reaction to start - enzymes help molecules collide successfully and therefore reduce the activation energy
high temperature and high pH increase kinetic energy and speed - this increases the number of successful collisions and the rate of reaction
activation energy needs to be supplied for a reaction to start - enzymes help molecules collide successfully and therefore reduce the activation energy
10
New cards
how is the rate of chemical reactions increased?
increase concentration - more frequent collisions
increase temperature - more frequent and successful reactions
increase surface area - more frequent collisions
add a catalyst - reduces activation energy required
increase temperature - more frequent and successful reactions
increase surface area - more frequent collisions
add a catalyst - reduces activation energy required
11
New cards
what are the two enzyme models?
lock and key theory (old model)
induced fit model (new model)
induced fit model (new model)
12
New cards
what is lock and key theory?
only one substrate (key) can fit into the enzyme’s active site (lock) - the shape of the active site is complementary to the substrate
when the substrate binds to the active site an enzyme-substrate complex is formed
the product is then released and the enzyme is unchanged
when the substrate binds to the active site an enzyme-substrate complex is formed
the product is then released and the enzyme is unchanged
13
New cards
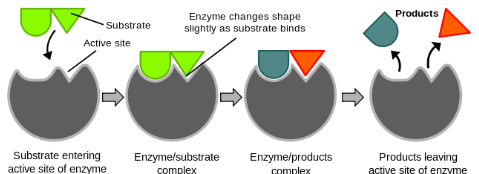
what is the induced fit model?
the active site and substrate are not complementary
instead the active site is flexible and changes shape which allows the substrate to fit
an enzyme-substrate complex is formed
the product moves away as it has become a different shape and the enzyme returns to its original shape
instead the active site is flexible and changes shape which allows the substrate to fit
an enzyme-substrate complex is formed
the product moves away as it has become a different shape and the enzyme returns to its original shape
14
New cards
what are intracellular enzymes?
made and retained inside the cell
15
New cards
what are intracellular enzymes involved in?
lysosomal enzymes - intracellular digestion of cellular components and foreign matter
metabolic pathways - e.g. aerobic respiration, photosynthesis, protein synthesis
polymerases - DNA replication and transcription
ATPase - hydrolysis of ATP to ADP and Pᵢ to release energy for vital biochemical processes
phagocytosis
metabolic pathways - e.g. aerobic respiration, photosynthesis, protein synthesis
polymerases - DNA replication and transcription
ATPase - hydrolysis of ATP to ADP and Pᵢ to release energy for vital biochemical processes
phagocytosis
16
New cards
what are extracellular enzymes?
work outside of the cell
many polymers are too large to enter the cell and must be broken down before entering
many polymers are too large to enter the cell and must be broken down before entering
17
New cards
what are extracellular enzymes involved in?
amylase - works outside of the cells in saliva, catalyses the breakdown of starch to maltose, is a single chain of amino acids with both alpha helix and beta pleated sheet sections
trypsin - type of protease, produced by pancreatic cells and released into the small intestine, catalyses the hydrolysis of peptide bonds of large polypeptides into smaller polypeptides which eventually get broken down into amino acids
trypsin - type of protease, produced by pancreatic cells and released into the small intestine, catalyses the hydrolysis of peptide bonds of large polypeptides into smaller polypeptides which eventually get broken down into amino acids
18
New cards
what factors affect the rate of enzyme controlled reactions?
temperature
pH
enzyme concentration
substrate concentration
pH
enzyme concentration
substrate concentration
19
New cards
how does temperature affect the rate of enzyme controlled reactions?
enzymes have an optimum temperature at which they catalyse reactions at the maximum rate - 37°C (body temperature)
20
New cards
how do lower temperatures affect the rate of enzyme controlled reactions?
lower temperatures prevent reactions or slow them down
molecules have less kinetic energy and move slower
there is a lower frequency of successful collisions, and less formation of enzyme-substrate complexes
substrates and enzymes collide with less energy - it is less likely that bonds will form or break
molecules have less kinetic energy and move slower
there is a lower frequency of successful collisions, and less formation of enzyme-substrate complexes
substrates and enzymes collide with less energy - it is less likely that bonds will form or break
21
New cards
how do higher temperatures affect the rate of enzyme controlled reactions?
high temperatures speed up reactions
molecules have more kinetic energy and move faster
there is a higher frequency of successful collisions, and more formation of enzyme-substrate complexes
substrates and enzymes collide with more energy - it is more likely that bonds will form or break
however, as temperature increases past the optimum temperature, the enzyme begins to denature and the rate of reaction drops sharply
the tertiary structure changes - bonds holding the enzyme together start to break
this permanently damages the active site, preventing substrates from bonding
molecules have more kinetic energy and move faster
there is a higher frequency of successful collisions, and more formation of enzyme-substrate complexes
substrates and enzymes collide with more energy - it is more likely that bonds will form or break
however, as temperature increases past the optimum temperature, the enzyme begins to denature and the rate of reaction drops sharply
the tertiary structure changes - bonds holding the enzyme together start to break
this permanently damages the active site, preventing substrates from bonding
22
New cards
what is a temperature coefficient?
Q₁₀
represents how much the rate of reaction changes when the temperature increases by 10°C
value is usually between 2 and 3
represents how much the rate of reaction changes when the temperature increases by 10°C
value is usually between 2 and 3
23
New cards
how are temperature coefficients calculated?
Q₁₀ = rate of reaction at temperature X + 10°C / rate of reaction at temperature X
24
New cards
how does pH affect the rate of enzyme controlled reactions?
all enzymes have an optimum pH
each enzyme’s optimum pH is different
each enzyme’s optimum pH is different
25
New cards
what happens to enzymes at extreme pH?
enzymes are denatured at extreme pH
the H bonds and ionic bonds that hold the tertiary structure together break
this alters the shape of the active site and prevents substrates from binding
the H bonds and ionic bonds that hold the tertiary structure together break
this alters the shape of the active site and prevents substrates from binding
26
New cards
how does enzyme concentration affect the rate of enzyme controlled reactions?
increased enzyme concentration increases the number of active sites available for binding
increases the likelihood of enzyme-substrate complex formation
as long as there is sufficient substrate available, rate of reaction increases linearly
increases the likelihood of enzyme-substrate complex formation
as long as there is sufficient substrate available, rate of reaction increases linearly
27
New cards
how does substrate concentration affect the rate of enzyme controlled reactions?
increased substrate concentration increases the likelihood of enzyme-substrate complex formation
28
New cards
what happens if the enzyme concentration is fixed and more substrate is added?
if enzyme concentration is fixed and more substrate is added - all active sites will become saturated
when all active sites are saturated, substrate molecules that are added will have nowhere to bind
when all active sites are saturated, substrate molecules that are added will have nowhere to bind
29
New cards
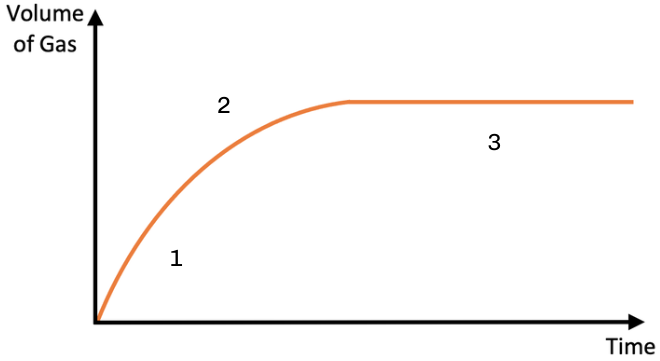
what does the rate of reaction graph show?
1) rapid initial rate of reaction - lots of substrate molecules, frequent successful collisions
2) reaction slowing down - less substrate molecules, less chance of successful collisions
3) reaction has stopped - no substrate molecules left to collide with the active site
2) reaction slowing down - less substrate molecules, less chance of successful collisions
3) reaction has stopped - no substrate molecules left to collide with the active site
30
New cards
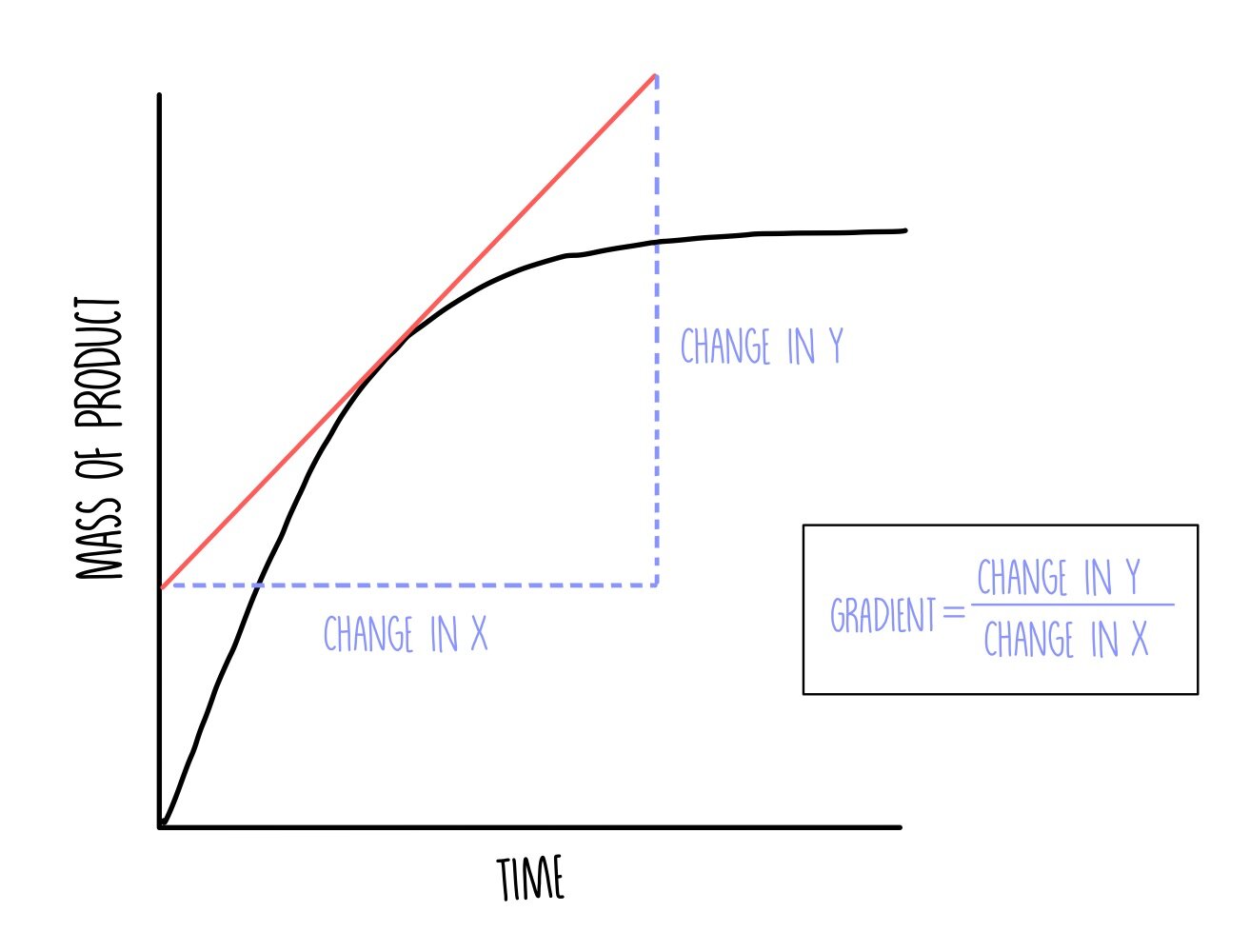
how is rate of reaction measured on a graph?
draw a tangent at the point which needs to be measured
draw a triangle
divide the height by the length - y/x
draw a triangle
divide the height by the length - y/x
31
New cards
how is rate of reaction calculated?
rate of reaction = 1/time
time must be in seconds
time must be in seconds
32
New cards
what are the two types of enzyme inhibitors?
competitive inhibitors
non-competitive inhibitors
non-competitive inhibitors
33
New cards
where do competitive inhibitors bind?
active site
34
New cards
how do competitive inhibitors reduce rate of reaction?
compete with substrates for the active site of the enzyme
whilst the competitive inhibitor is bound to active site substrates cannot collide with it and form enzyme-substrate complexes - reduces the rate of reaction
whilst the competitive inhibitor is bound to active site substrates cannot collide with it and form enzyme-substrate complexes - reduces the rate of reaction
35
New cards
what is reversible competitive inhibition?
after a short time, the competitive inhibitor leaves the active site
36
New cards
can the effects of competitive inhibitors be overcome?
the effects of competitive inhibitors can be overcome by increasing the substrate concentration - creates a greater chance of a successful collision between the active site and a substrate
37
New cards
where do non-competitive inhibitors bind?
allosteric site
38
New cards
how do non-competitive inhibitors reduce the rate of reaction?
cause the tertiary structure to change - alters the shape of the active site so it is no longer complementary to the substrate
the substrate can no longer bind to the active site so rate of reaction is reduced
the substrate can no longer bind to the active site so rate of reaction is reduced
39
New cards
can the effects of non-competitive inhibitors be overcome?
the effects of competitive inhibitors cannot be overcome by increasing the substrate concentration - even if substrate concentration is increased, the substrate molecules still cannot bind with the active site
40
New cards
what are the three types of cofactors?
cofactors
coenzymes
prosthetic group
coenzymes
prosthetic group
41
New cards
what all cofactors do?
enhance enzyme function
usually help to form the active site, but can bind to other parts of the enzyme
usually help to form the active site, but can bind to other parts of the enzyme
42
New cards
what is the difference between cofactors and coenzymes?
cofactors are inorganic - e.g. zinc, iron, copper ions
coenzymes are organic - e.g vitamins, NAD (a large complex molecule made from vitamin B3 involved in respiration)
coenzymes are organic - e.g vitamins, NAD (a large complex molecule made from vitamin B3 involved in respiration)
43
New cards
what can happen if cofactors are damaged?
cofactors can be disabled or replaced by some substances - e.g. mercury
this can inhibit enzyme function
this can inhibit enzyme function
44
New cards
what is a prosthetic group?
cofactors that become a permanent part of the enzyme’s structure