Corrosion & Degredation of Materials
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Corrosion Process
Electrochemical such that there is a transfer of electrons from one chemical species to another
Reaction is from anode (oxidation) to Cathode (reduction)
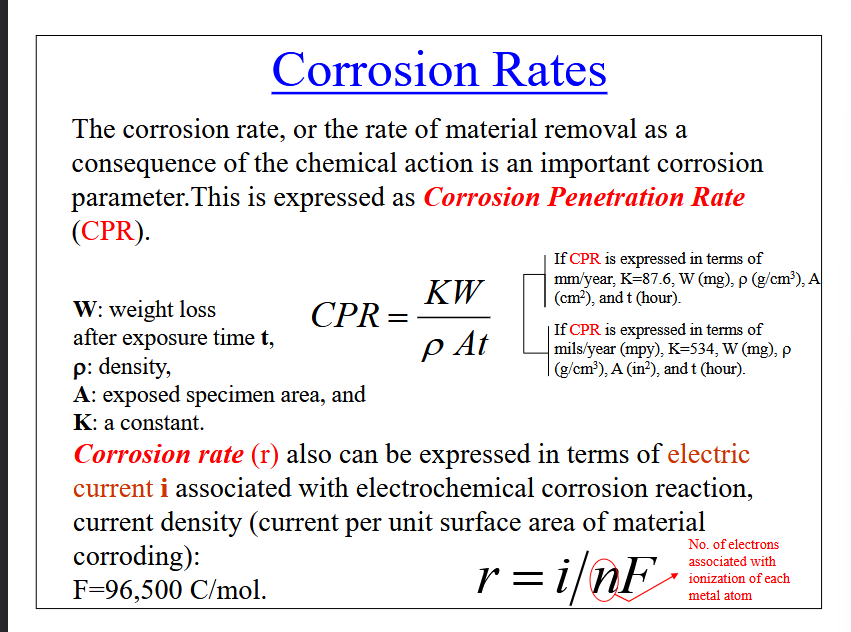
Corrosion Penetration Rate (CPR)
The rate at which material is removed as a consequence of the chemical reaction
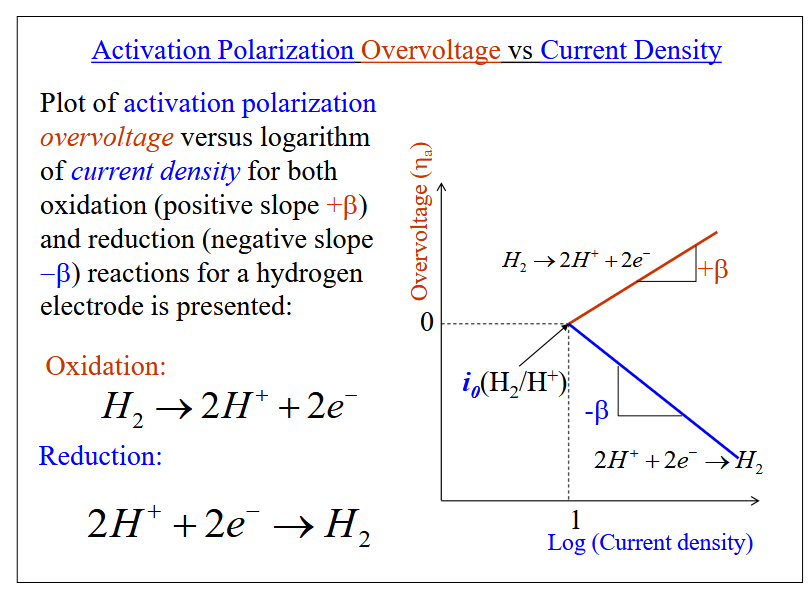
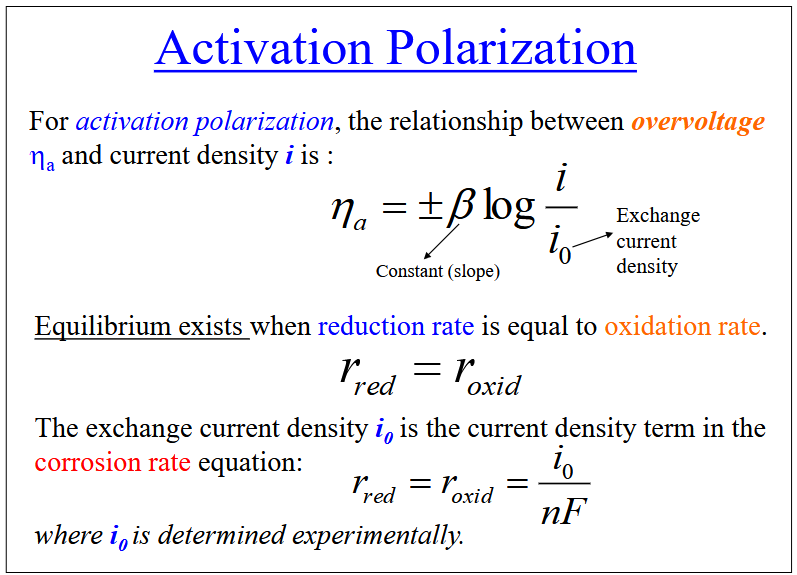
Polarization
The displacement of each electrode potential from its equilibrium value. The magnitude of this displacement is the overvoltage
Activation polarization: The rate determining step is the step with the lowest rate
Influence of Concentration and Temperature on Cell-Potential
The EMF series only applies to pure metals in 1 Molar solution at 25 degrees celcius
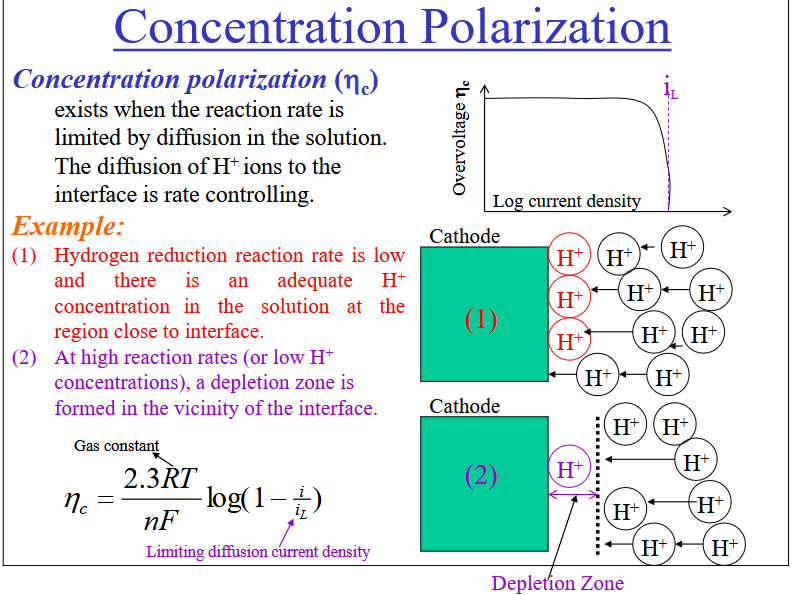
Concentration Polarization
The reaction rate is limited by diffusion in the solution
Passivity
Some metals and alloys lose their chemical reactivity and become extremely inert. Results from the formation of a thin oxide film on the metal surface.
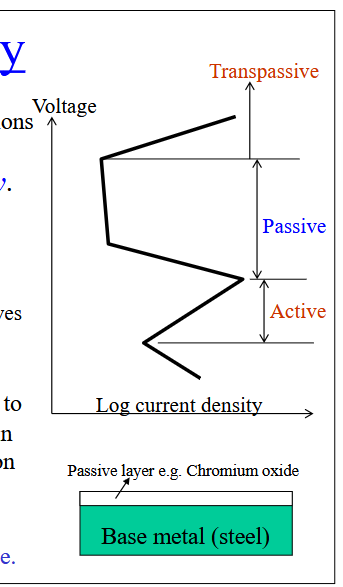
Parameters Damaging Passivity
High temperature
salt
High voltage
Uniform Corrosion
Electrochemical corrosion that occurs with equivalent intensity over the entire exposed surface, often leaving behind a scale of deposit.
Galvanic Corrosion
Occurs when two metals or alloys with different compositions are electrically couples while exposed to an electrolyte. The more reactive metal (anode) will experience corrosion, the less reactive metal (cathode) will not.
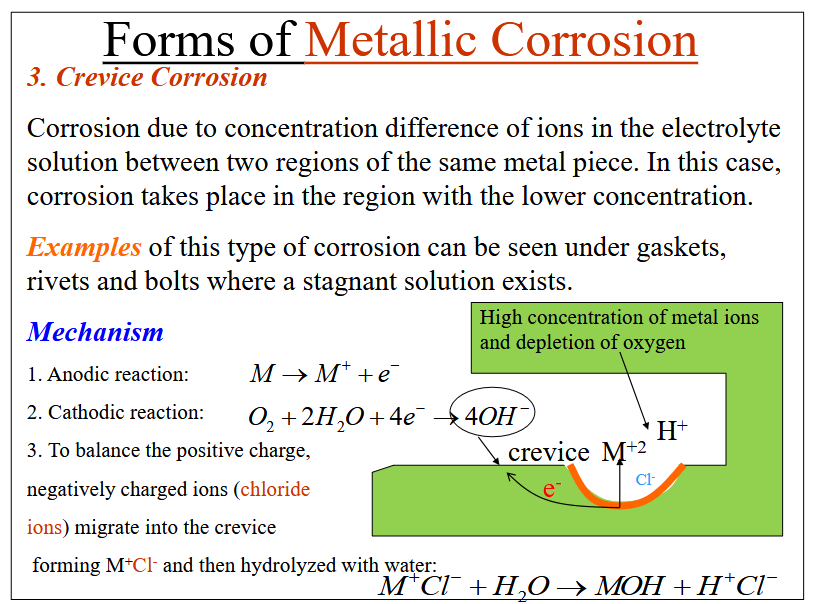
Crevice Corrosion
Corrosion due to the difference of ions in the electrolyte solution between two regions of the same metal piece. Corrosion occurs in the region with the lower concentration
Pitting Corrosion
A form of very local corrosion in which small pits or holes form

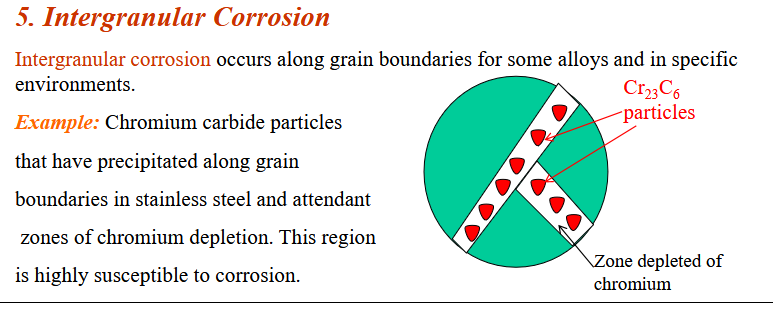
Inter-granular Corrosion
Corrosion along the grain boundaries
Selective Leaching Corrosion
Occurs when one element is purposely removed during corrosion.
Erosion - Corrosion
The combination of chemical attack and mechanical abrasion/wear as a consequence of fluid motion. Commonly found in pipe bends, elbows, and abrupt changes in pipe diameter position.
Occurs when fluid changes its direction of flow, suddenly becoming turbulent
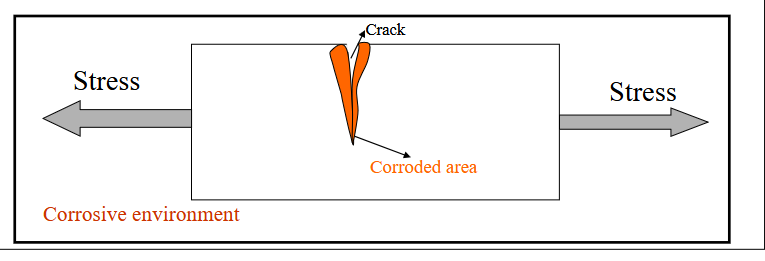
Stress Corrosion
Results from the combined action of an applied tensile stress and a corrosive environment. Small cracks form and then propagate in a direction perpendicular to the direction of stress.
Results in failure
Hydrogen Embrittlement (Hydrogen Stress Cracking)
When an atom of hydrogen penetrates into steel causing a significant reduction in ductility and tensile strength.
H-atoms diffuse interstitially through the crystal lattice, leading to cracking, usually transgranular