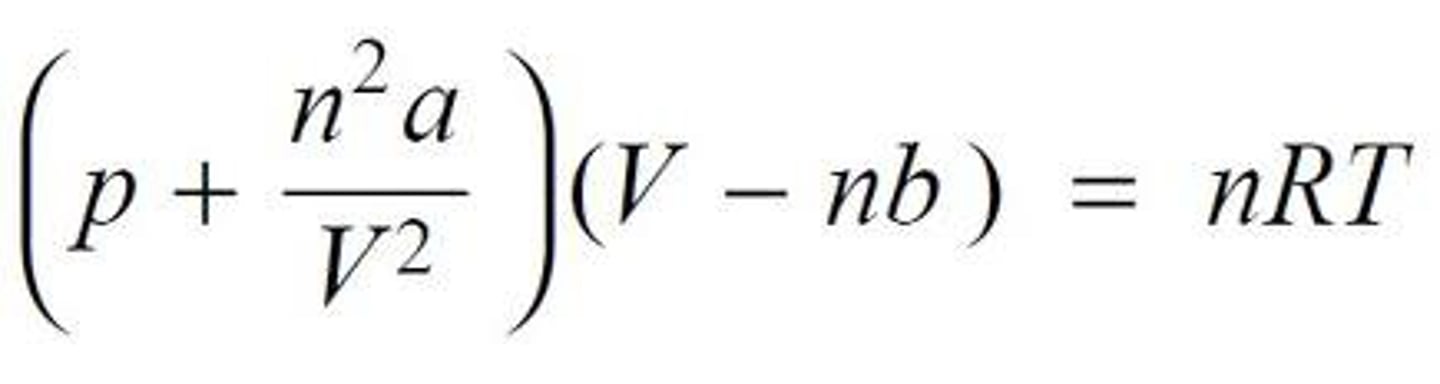CH 5 Gases & Kinetic-Molecular Theory
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Liquid
Variable shape, constant volume; particles move freely around each other
Solid
Fixed shape and volume; particles tightly packed.
Gas
Variable shape and volume; particles far apart & move randomly
volume changes significantly w/ P & T
atoms can be squeeze together unlike solids/liquids
expand when heated/shrink when cooled
Form solutions in many proportions (not fixed)
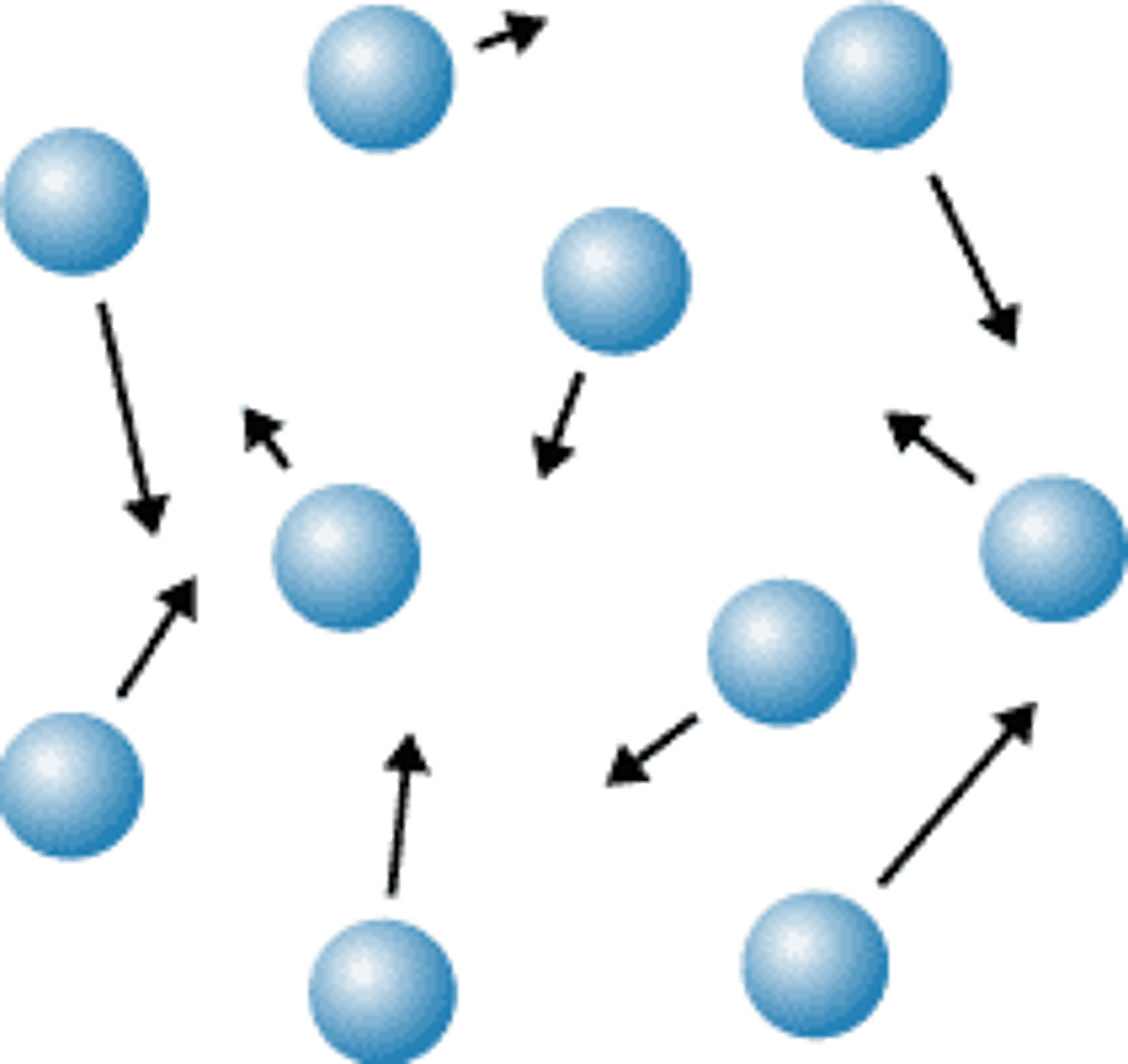
Pressure from a gas...
Force from gas particles colliding with container walls.
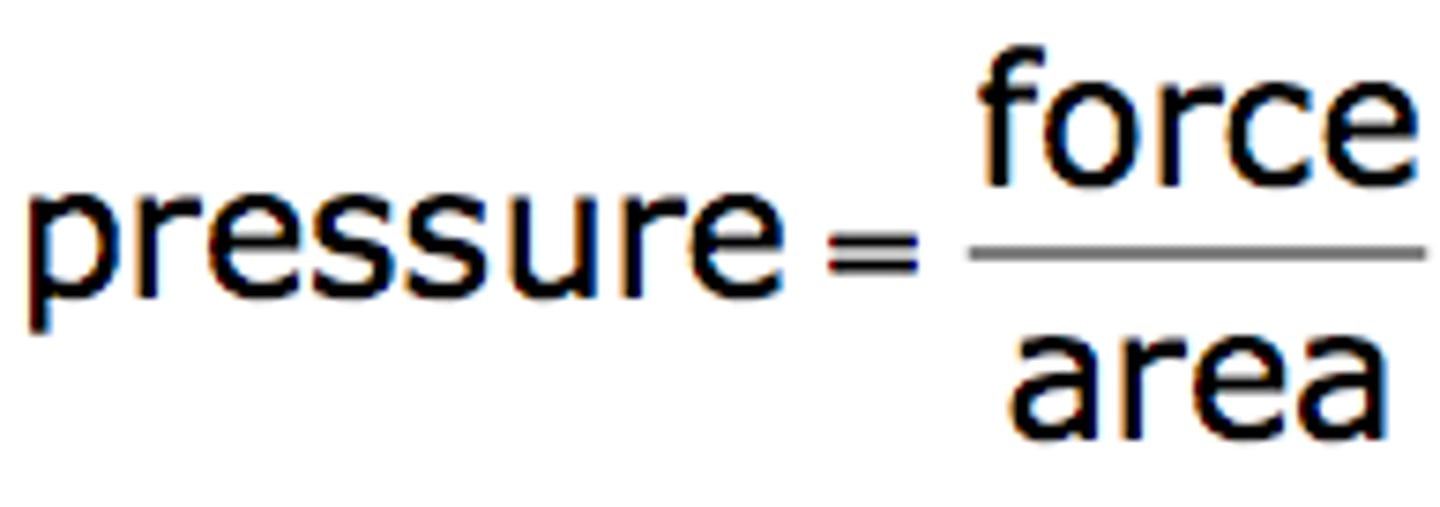
Atmospheric Pressure
arises from mass exerted by all atmospheric gases onto Earth's surface = ~14.7 lb/in^2 (or psi)
gases constantly surrounding us do have mass, and it's always pushing down on everything
decreases w/ altitude (further you go, less gas above you)
Common Units of Pressure
1 atm
760 mmHg
760 torr
Exact numbers!!!
Ideal Gas
gas exhibits linear relationships among P, T, V, & n
no ideal gas actually exists, but most simple gases behave nearly ideal at ordinary T & P
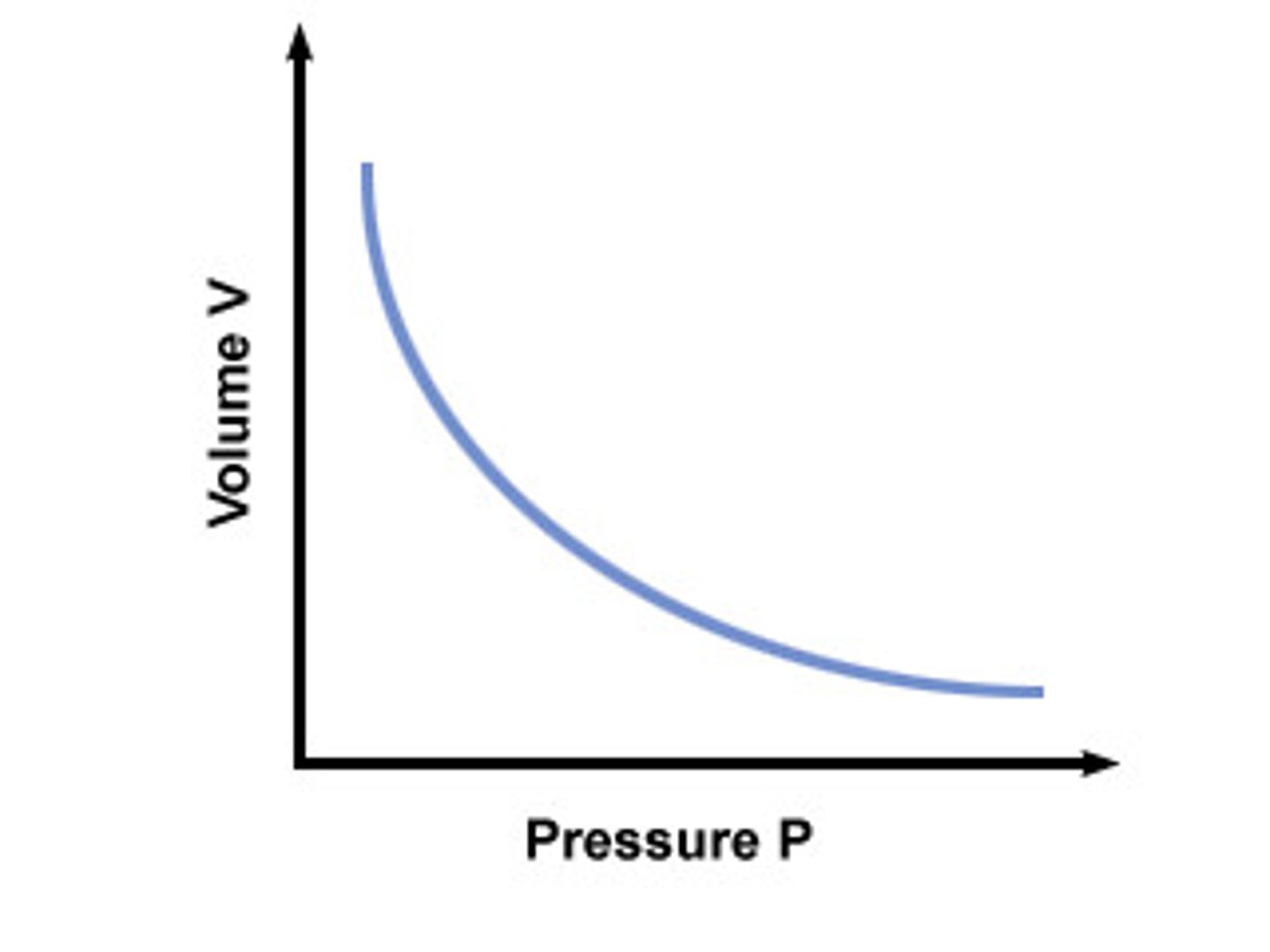
Boyle's Law
(V & P) at constant temp; the volume of fixed amount of gas is inversely proportional to external pressure
(as volume decrease pressure increases b/c there’s less room for gas and more collisions with the walls)
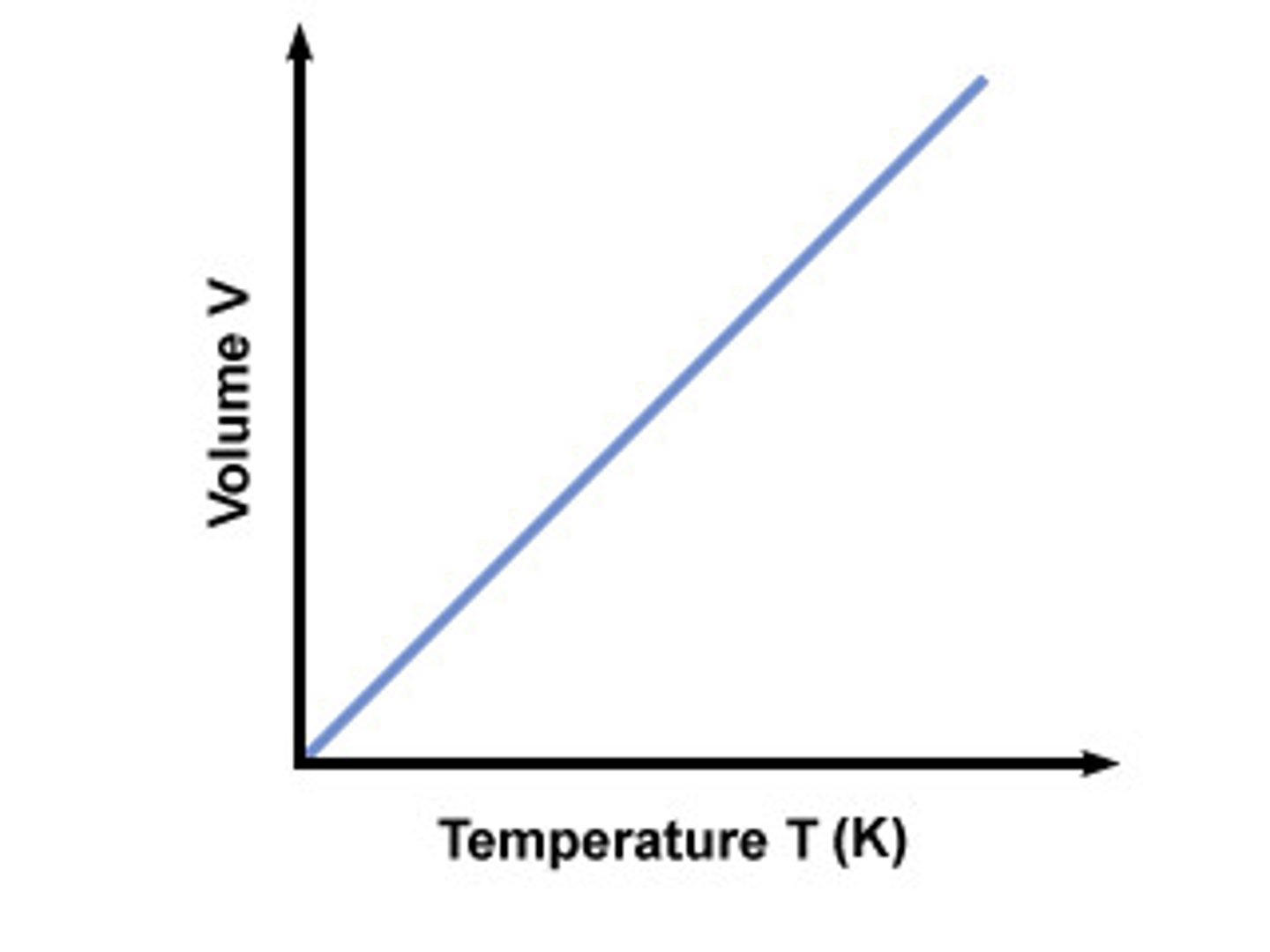
Charles's Law
at constant pressure; volume of fixed amount of gas is directly proportional to absolute (K) temp.
(if volume decrease there’s less room for a gas, causing more collision on the walls and higher pressure)
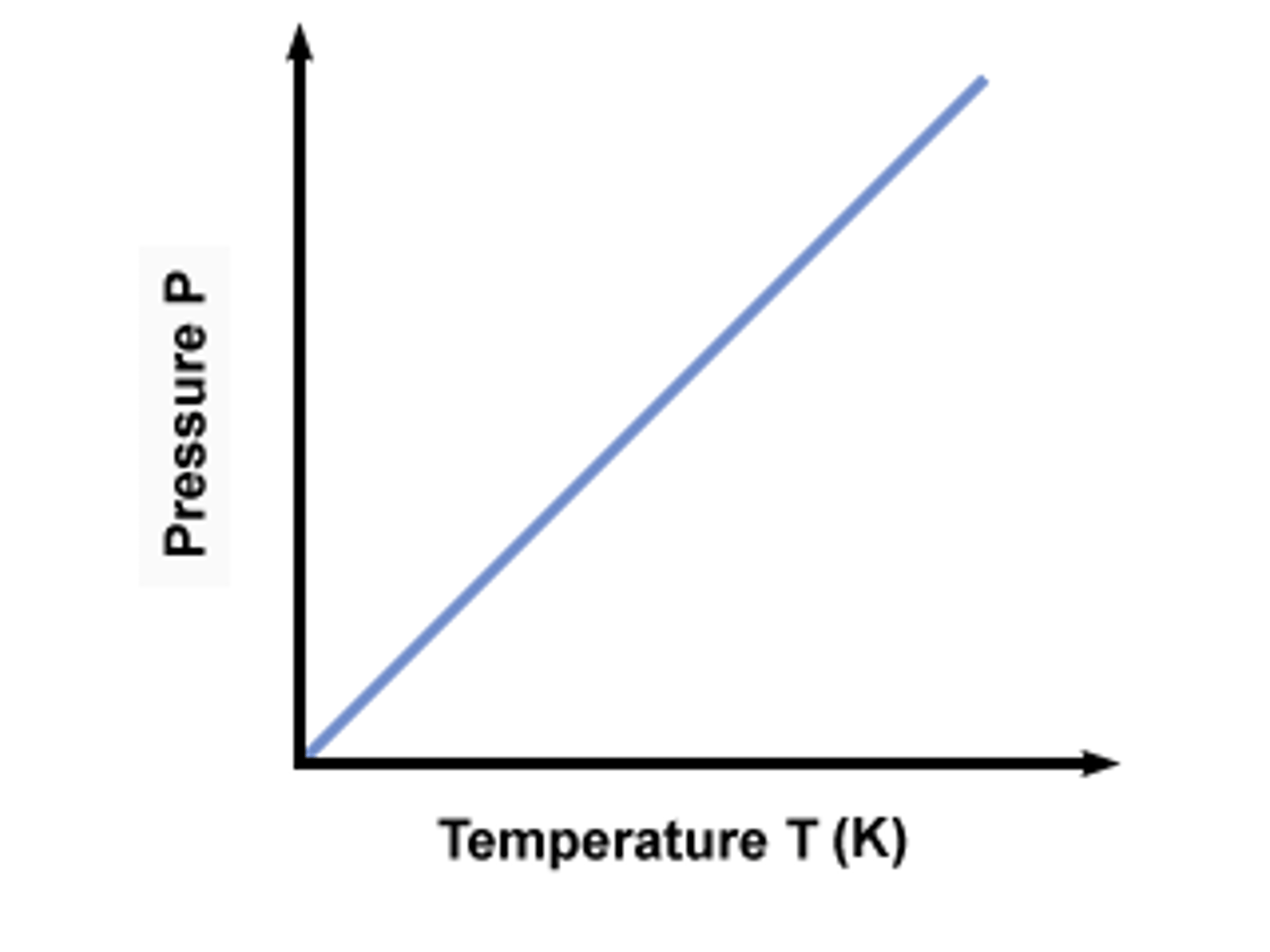
Gay Lussac's Law
(P & T) at constant volume; pressure exerted by fixed amount of gas is directly proportional to absolute (K) temp.
(higher temps cause more particle movement and energy, causing more collisions with the walls and greater pressure)
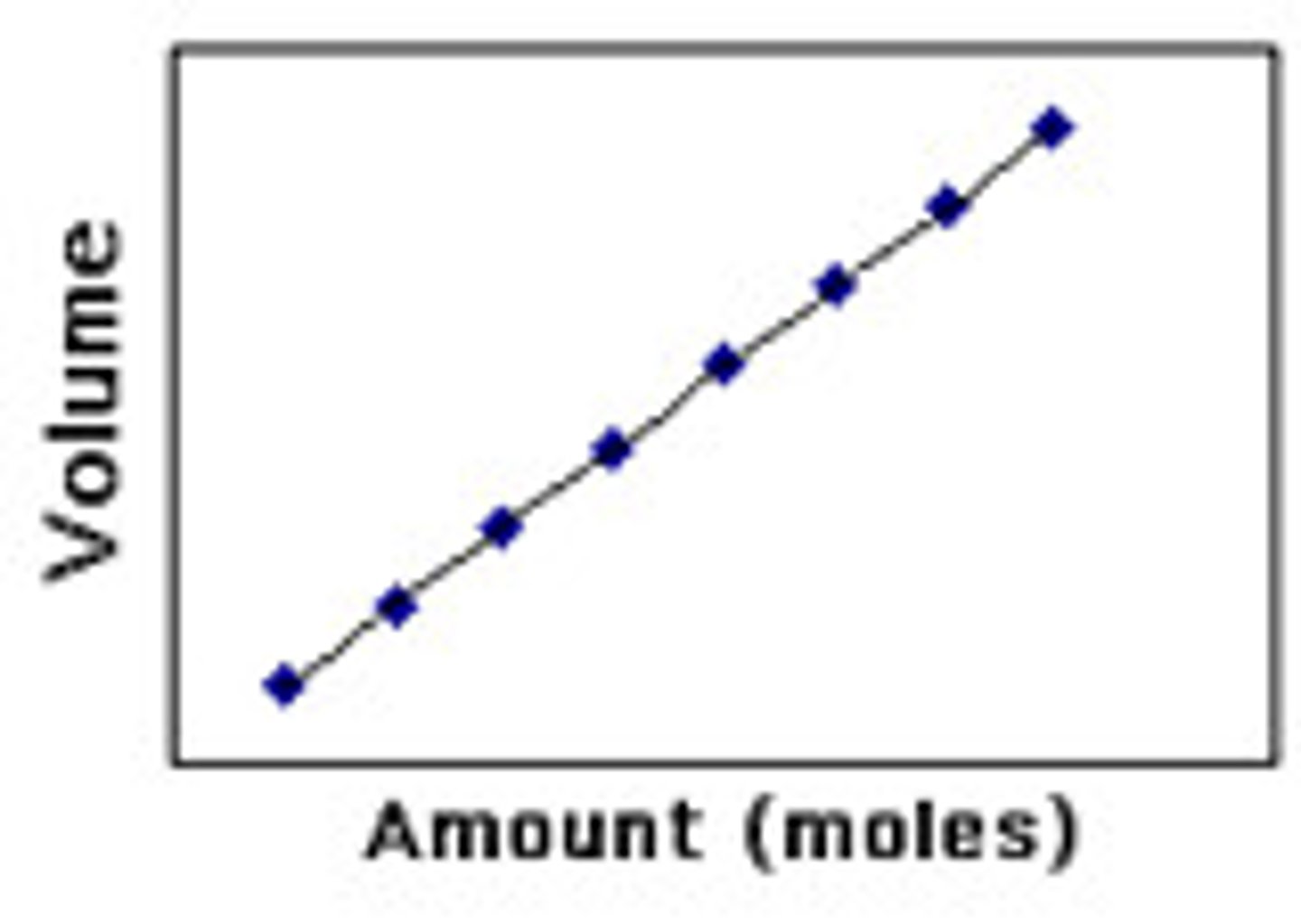
Avogadro's Law
(V & n) at fixed temp. & pressure; volume occupied by gas is directly proportional to amount of gas (n)
At fixed T & P any ideal gas with the same volume with have the same numbers of moles/particles
(more particles = more frequency of particle collisions and greater volume)
Ideal Gas Law
most gas laws have volume, so they relate to each other
R= constant-> must have all units in correct terms (1 atm22.414)/(1 mol273.15K) = .0821
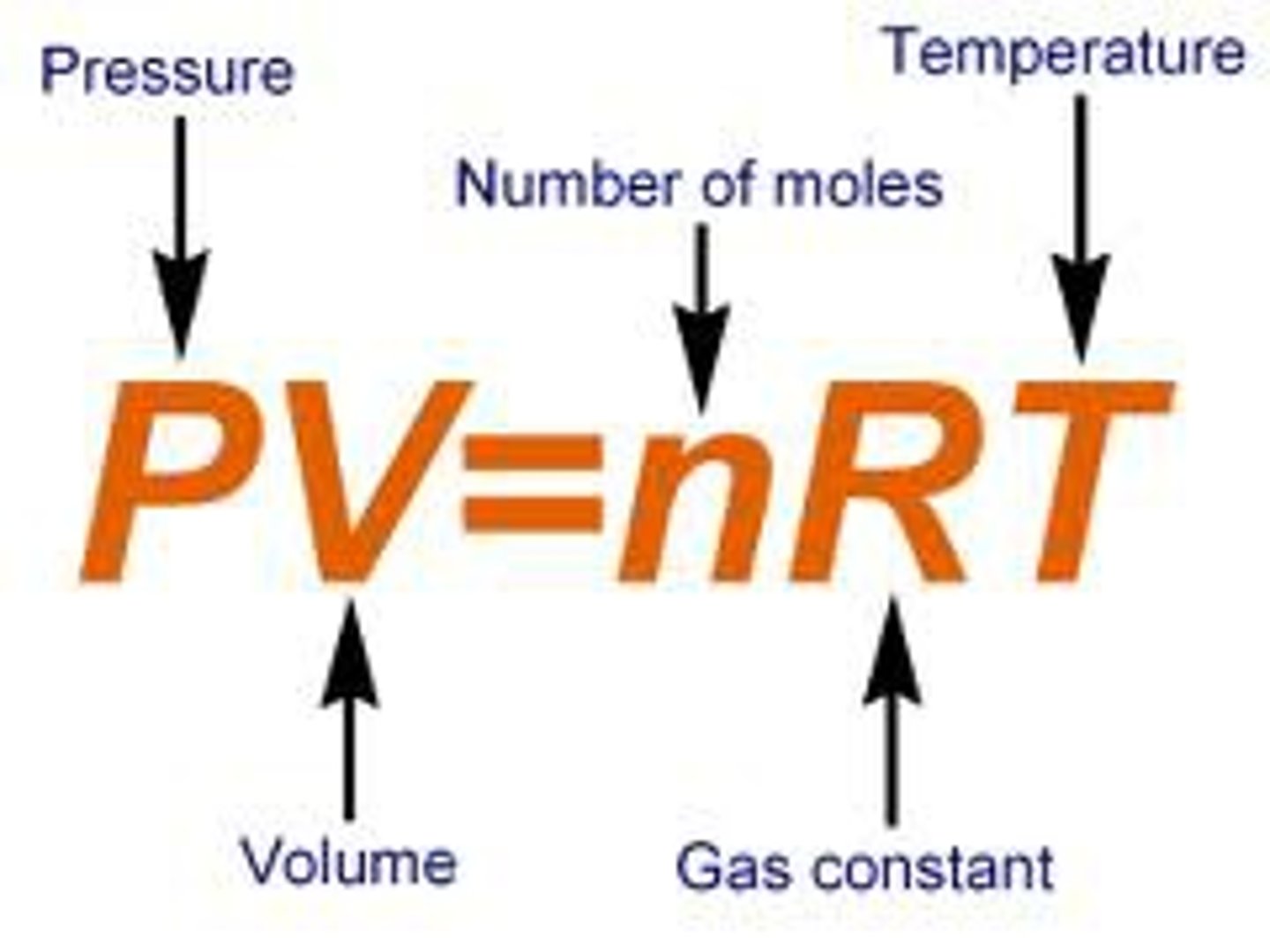
Ideal Gas Law for Calculations
(PV)/(nRT) = (PV)/(nRT)
Units that values of ideal gas law need to be in?
Pressure (P) in atm
Temperature (T) in K
Volume (V) in L
Amount/# of moles (n) in moles
Kinetic-Molecular Theory
Particle Volume
Gas particle are tiny, w/ large space b/w them. Volume of each particle is so small compared to total V of gas, it's assumed to be zero
Total volume of gas is total volume of container, aka ignore volume of individual gas particles
Particle Motion
Gas particles are constant, random, straight-line motion exp. When they collide w/ each other & container walls
Particle Collisions
Collisions are elastic (colliding particles exchange energy but do not lose any energy due to friction) B/w collisions, particles do not influence each other by attractive/repulsive forces
Gas Density
Derived based on ideal gas law (mass/volume)
PV/nRT can be used to get moles per liter, then converted to grams per liter
Gas Mixtures
Gases can form solutions in various proportions.
- all gases exert partial pressure, that added together equal total pressure of mixture
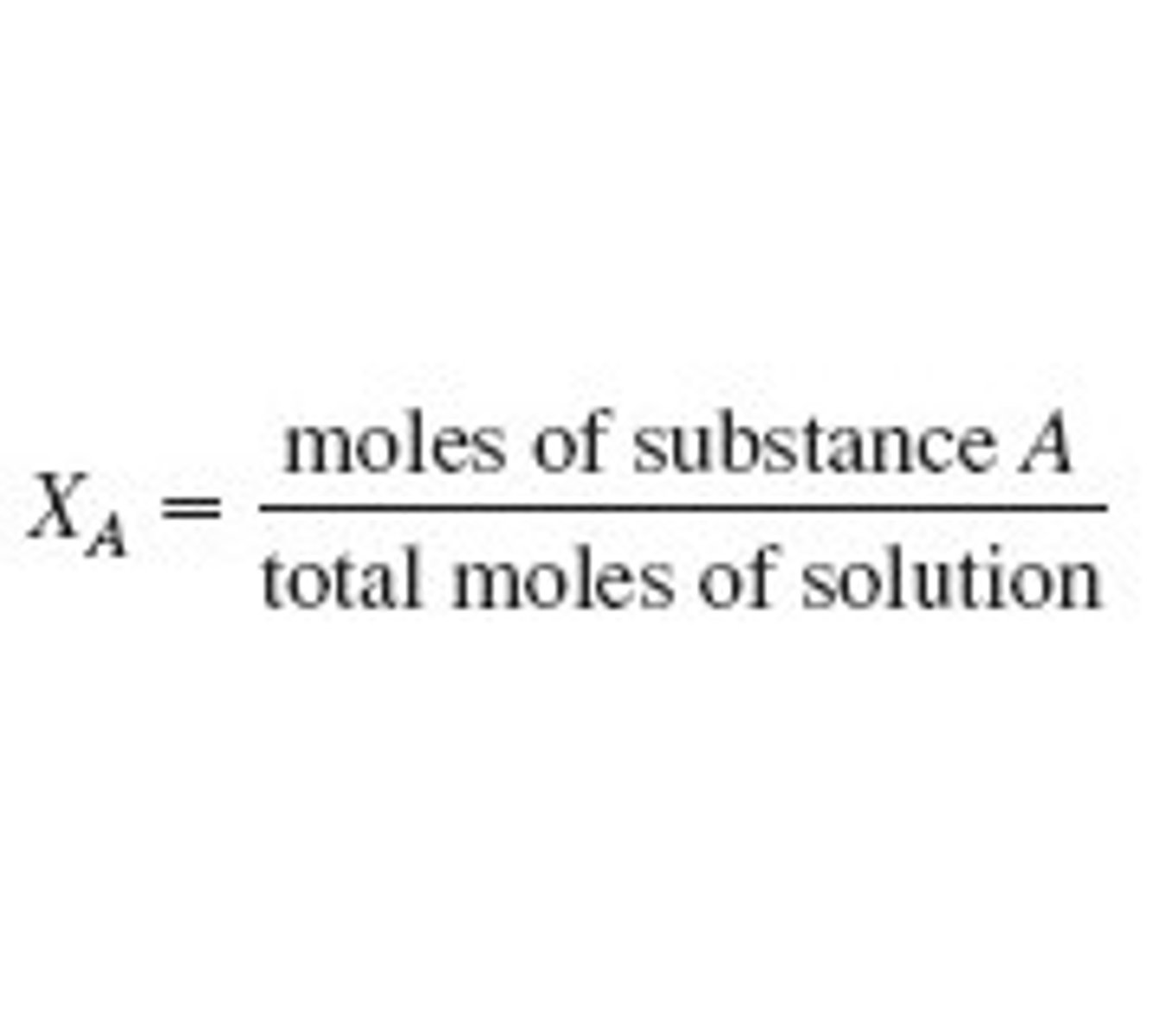
use mole fraction of gas→ moles gas/ total moles
partial pressure→ mole fraction * total pressure
Standard Temperature and Pressure (STP)
1 atm pressure and 0°C (273.15 K) temperature.
Standard Molar Volume
Volume of 1 mol of gas at STP is 22.414 L.
Dalton's Law
states that with partial pressure, the total pressure of mixture is sum of partial pressures of component gases
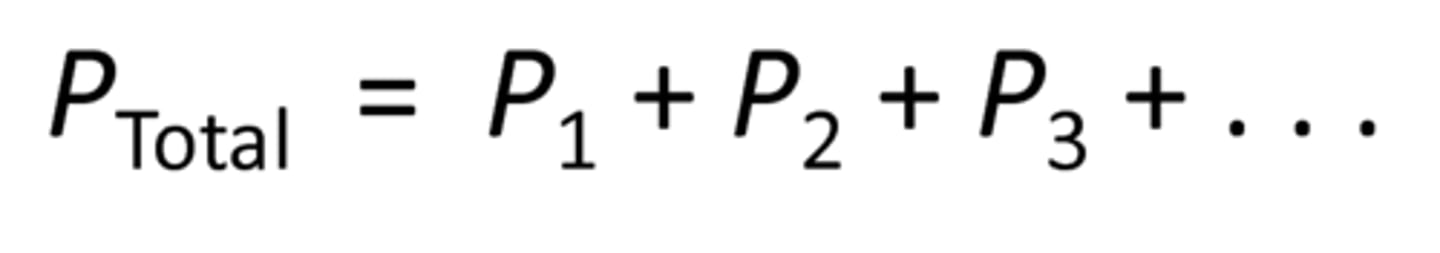
partial pressure of gas
use mole fraction of gas→ moles gas/ total moles
partial pressure→ mole fraction * total pressure
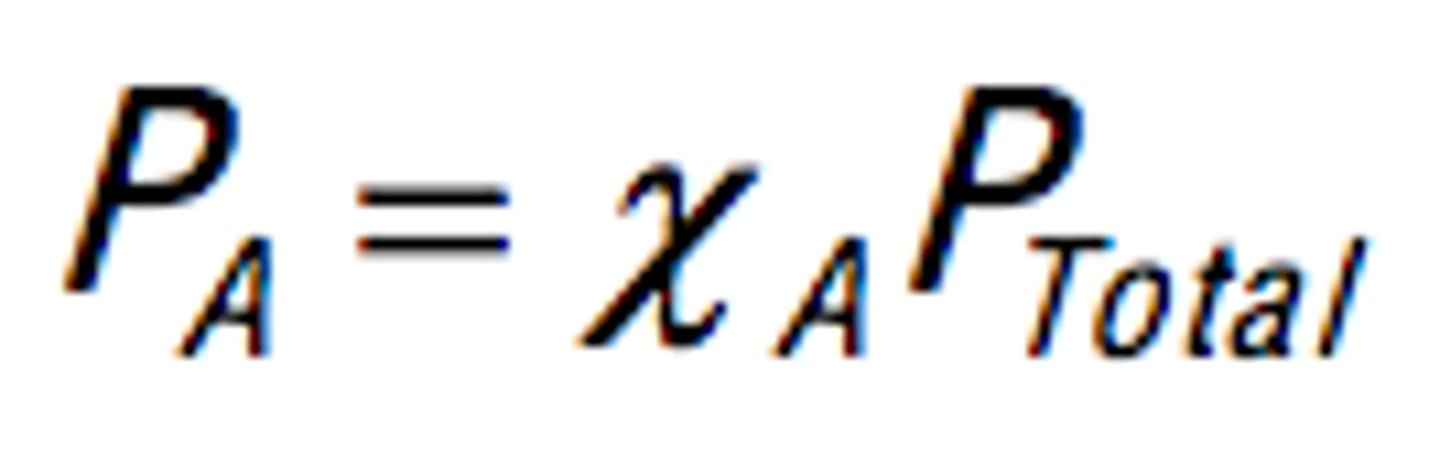
Mole Fraction
Ratio of partial pressure of gas to total pressure.
use mole fraction of gas→ moles gas/ total moles
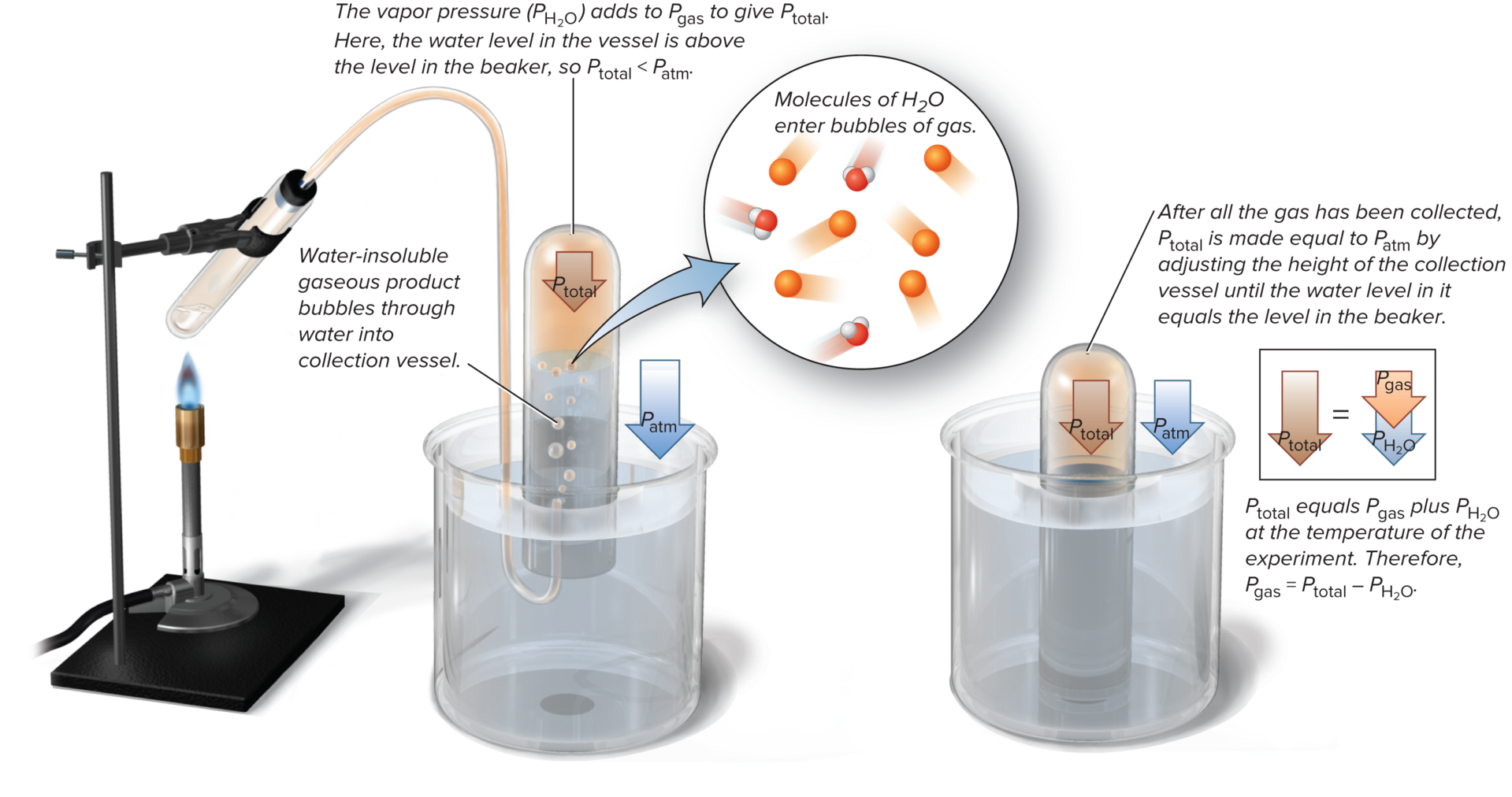
Vapor Pressure & reason behind it?
Whenever a gas is in contact with water, some of the water vaporizes into a gas as well
water vapor mixes with the gas contributing pressure
This portion of total pressure depends only on temperature of water
(Total pressure - vapor pressure) = gas pressure
Kinetic Energy
Average kinetic energy is equal for all gases at a given temperature.
Kinetic energy depends on both mass & speed of particle
At same T, a heavier gas particle moves slower than lighter one, but still has the same average kinetic energy (think slopes of graph)
same amount of energy, different mass and speed (heaver = slower, lighter = faster BUT sam energy)
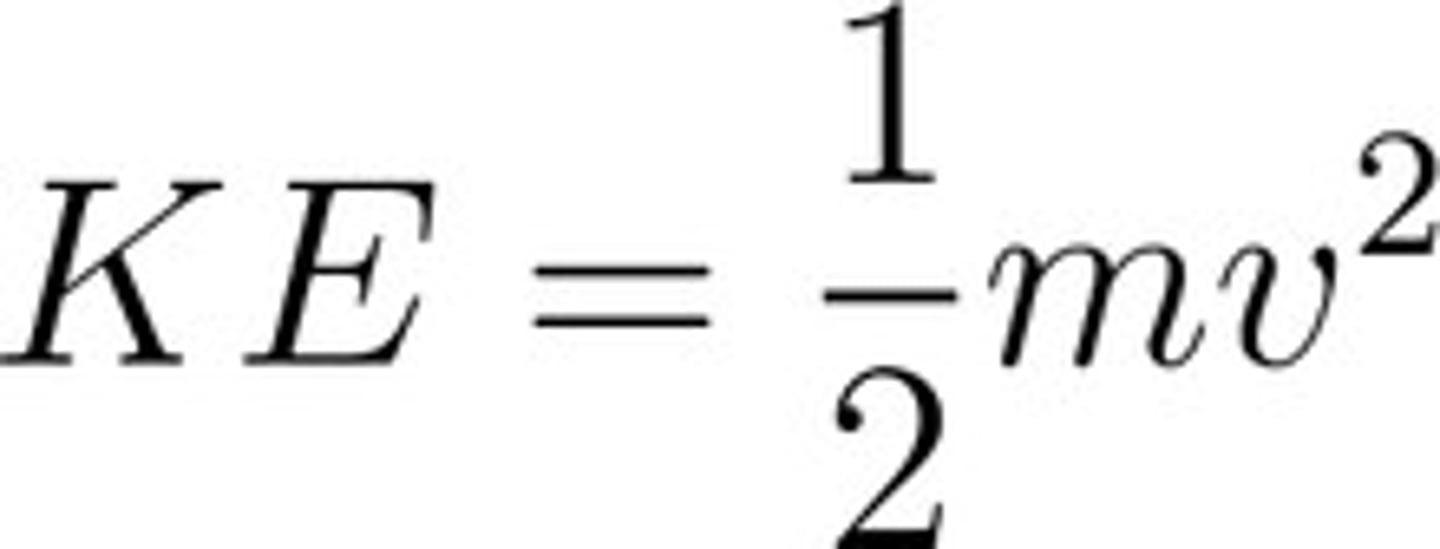
Relationship b/w Temperature & Energy
Directly proportional relationship with energy.
(all variables are constant except temperature in the equation)
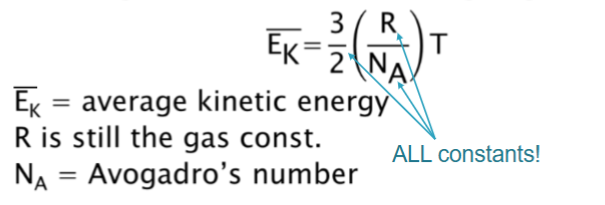
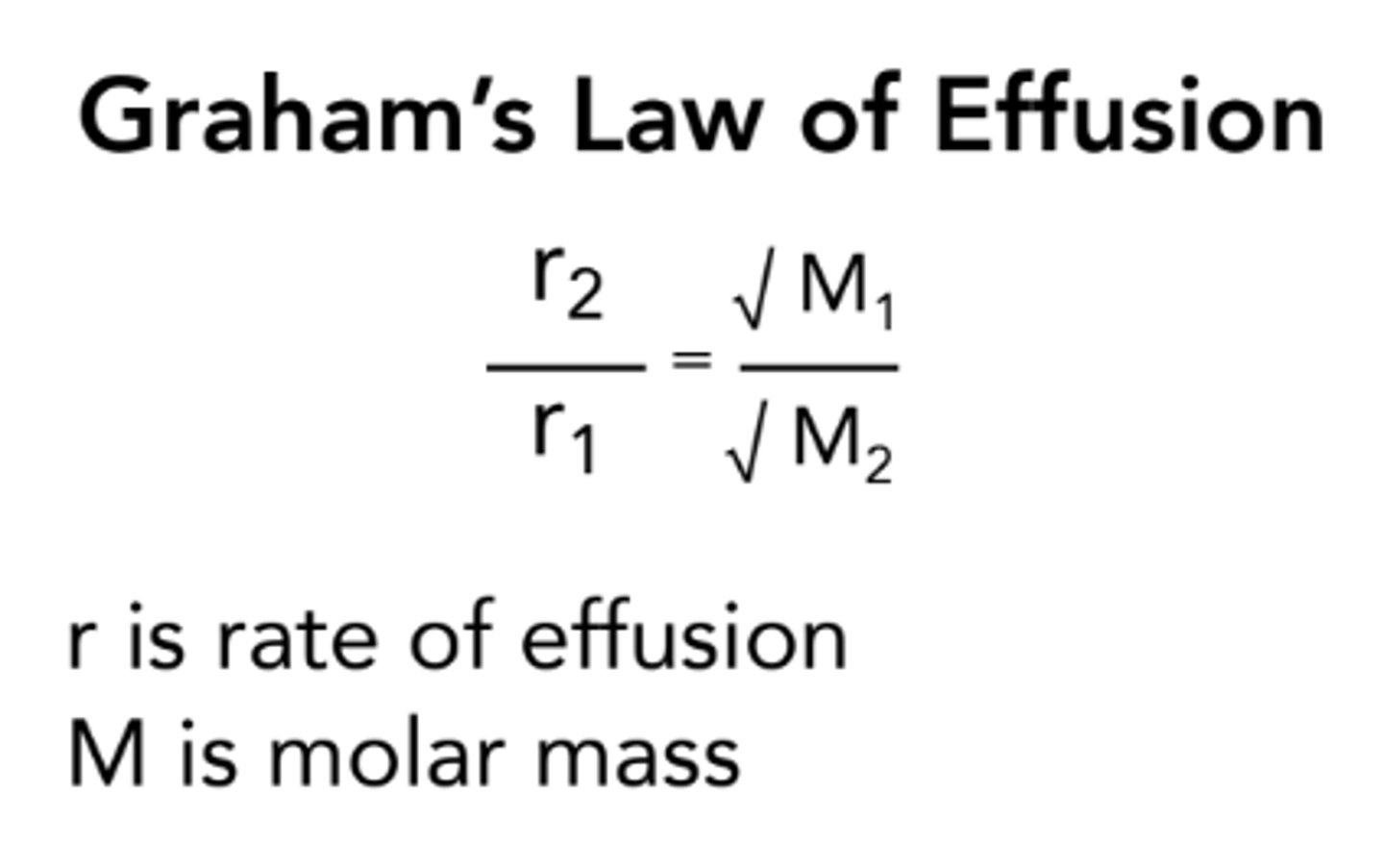
Graham's Law of Effusion
Rate of effusion of gas is inversely proportional to square root of its molar mass
Lighter gas moves faster-> higher rate of effusion than heavier gas at same T
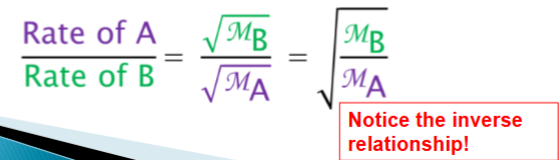
Graham’s Effusion w/ 2 Gases?
At given T & P, gas with lower MM moves faster than as w/ higher MM
More atoms of lighter gas reach hole & escape per unit of time
Constant T, the ration of effusion rates of two gases, A & B...
Effusion
Gas escaping through a small hole into a vacuum.
Diffusion
At given T & P, gas with lower MM moves faster than as w/ higher MM
- More atoms of lighter gas reach hole & escape per unit of time
Real Gases
Real gases have real volume
Gas particles are not points of mass, but have actual volumes associated with them
Real gases do experience attractive/repulsive forces b/w particles
Real gases deviate most from ideal behavior at low temperature & high pressure
Van der Waals Equation
Adjusts ideal gas law for real gas behavior.
Real volume of gas particles
Effect of interparticle attractions