Lecture #17 (just p53 info) and #18 | p53 - Protein Stability, Sensing DNA damage and DNA repair
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What is p53?
tumor suppressor and transcription factor gene that codes for a protein crucial for regulating cell cycle and preventing cancer
CKIs are up-regulated by p53 in response to DNA damage
It has been said that p53 unregulated CKIs (inhibitors) in response to DNA damage. Why is that important?
this up-regulation inhibits further proliferation that can cause cancer
What is the correlation between p53 and Li-fraumeni cancer patients?
Li-fraumeni patients: a family with a very high rate of cancers of all different types
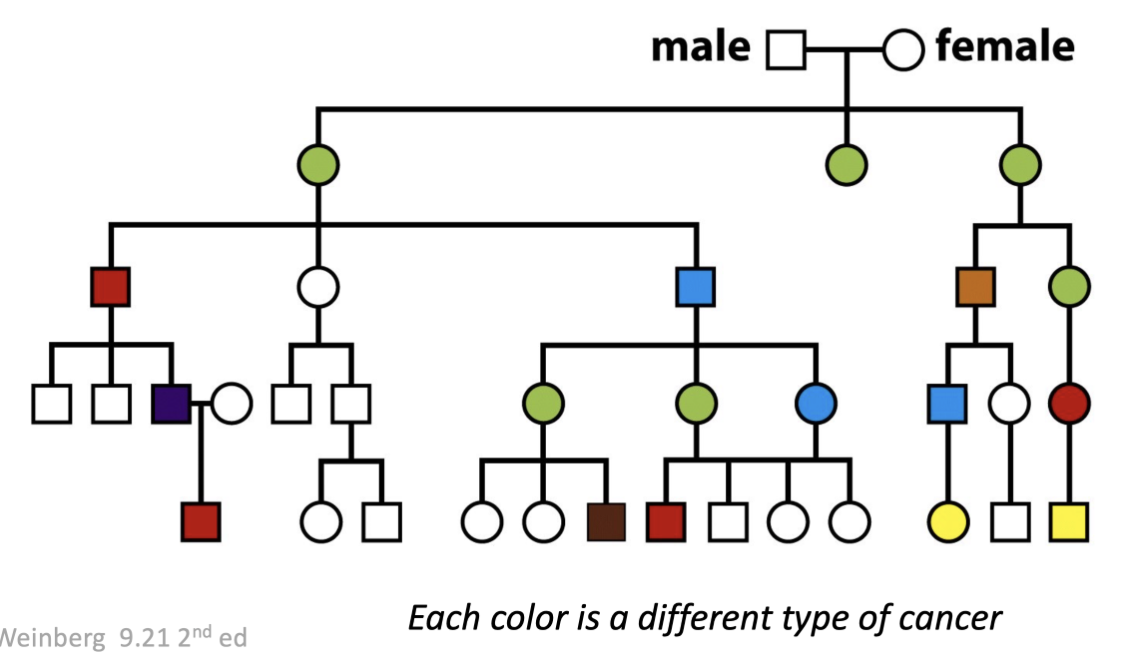
Found that in this group, p53 was mutated
more prone to development of cancer
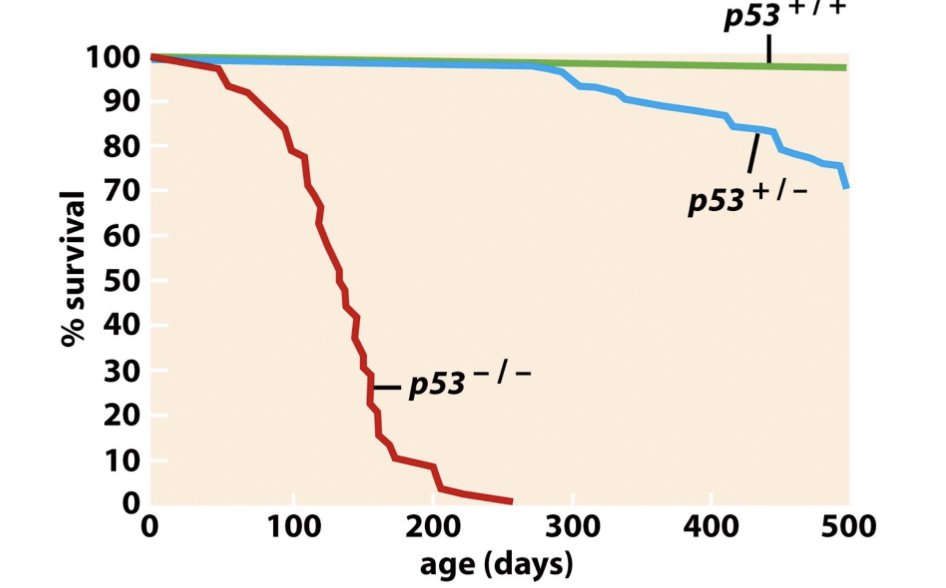
What happened in the experiment with p53 knocked out?
P53 knock out mice developed tumors early and survived much less that normal mice
Relation between p53 and human cancer
the most frequently mutated gene in human cancer
mutates in >50% of all human tumors and >90% in certain cancers
Is p53 a tumor suppressor or oncogene?
All properties indicate that p53 is a tumor suppressor
mutated in familial cancers
However first discoverers thought it was an oncogene
What early findings led researchers to think p53 was an oncogene?
p53 from transformed cell lines cooperates with activated Ras in transformed assay (this is the standard definition of an oncogene)
Injection of an anti-p53 monoclonal Ab into cells lead to growth arrest
Assumed that the Ab inhibited p53 function
Cells induced p53 when stimulated by growth factors
Expression of p53 peaks at G1/S which its consistent with role promoting S phase entry
What were the critical observations that determined that p53 was a tumor suppressor but not an oncogene?
1987: They were injecting virus into the p53 locus → inactivation of the p53 gene
1989: Mutant cDNA was used in previous studies which was the cDNA that was transforming Ras (indicating oncogene)
WT p53 ANTAGONIZED TRANSFORMATION
proven to not be an oncogene, but a tumor suppressor
p53 knockout mice were made and found to contain lots of tumors
What nicknames does p53 have?
Molecule of the year for 1993
“Guardian of the genome”
Cancer killer
Summary of p53
Most commonly mutated gene in human cancer
mutated in Li-Fraumeni Cancer Syndrome
Heritable
Has a very large consensus binding site (binding location for a specific protein or other molecule)
it is a transcription factor that binds a specific DNA sequence, recruits co-activators and activated txn
Represses transcription by recruiting HDAC to gene promoters
Increases the expression of CKI p21 gene
Normally present at low amounts in the cell; levels are increased upon DNA damage
How is p52 activated after DNA damage?
Damage occurs
p53 is activated by phosphorylation and it binds to the regulatory region of p21 (CKI)
Increases transcription of p21
p21 can bind to G1/S-Cdk and inactivate it so the e2f is kept under control
DNA damage → G1 arrest
How are p53 protein levels regulated?
MDM2 and ubiquitin
Phosphorylation by CHK2, ATM, ATR
Subcellular localization by p19-ARF
MDM2 overview
Murine tumors with double minute chromosomes
Binds p53 and targets it for destruction by directly sending p53 to the proteasome
MDM2 has E3 ubiquitin ligase activity
(the binding of ubiquitin leads to sending to proteasome → destroyed)
HOWEVER: If p53 is already phosphorylated at Ser 15, 20, 37 (meaning p53 is already activated) → MDM2 cannot bind and break it down
Describe the MDM2 mechanism to bind to p53
p53 binds DNA as a tetramer
MDM2 binds and polyubiquinates non phosphorylated p53
26S proteasome degrades p53
MDM2 has E3 ubiquitin ligase activity, meaning it can identify the p53 that needs to be targeted
What is the negative feedback loop between MDM2 and p53?
MDM2 is a target gene of p53, so the activation of p53 produces MDM2
increased levels of MDM2 leads to the degradation of unphosphorylated p53
so if p53 is mutated? MDM2 is not expressed and mutant p53 protein levels are elevated
Is MDM2 a tumor suppressor gene or oncogene?
Oncogene
mutated gene that has the potential to cause cancer
When is MDM2 amplified?
When p53 is not mutated
How do RB and p53 block cell cycle progression?
Rb: Represses Cell proliferation genes
p53: activates cell cycle arrest genes by increasing the production of CKI like p21 genes
Thus, viral proteins need to inactivate both mechanisms
Need to activate cell proliferation genes in Rb model
Need to repress cell cycle arrest apoptosis genes
What does different promoters in Ink4a do?
Express genes p16 and p19ARF
p19 ARF inhibits MDM2 → Increased p53 expression
How does p19 ARF antagonize the ability of MDM2 to export p53 from the nucleus to the cytoplasm for degradation?
p53/MDM2 shuttle between the nucleus and the cytoplasm
MDM2 targets p53 for destruction in the ectoplasm
expression of p19-ARF results in the formation of nuclear antibodies
p19-ARF sequesters MDM2 in the nucleolus, preventing it from exporting p53 for degradation
SO: expression of the p 19 tumor suppressor gene is stimulated by oncogenes like Myc
so could p19ARF be an anti-oncogene checkpoint
Layout: Rb can inhibit E2F and Myc, which stops the stimulation of p19 arf
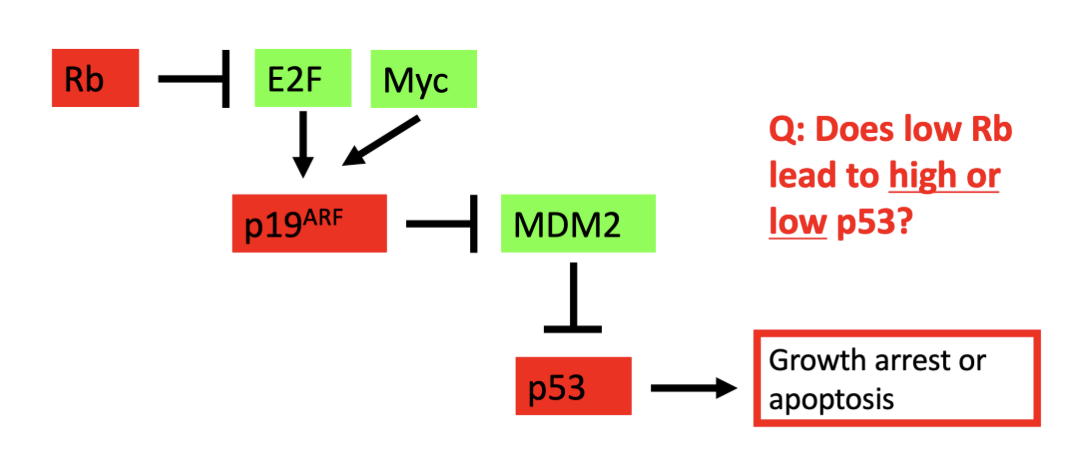
So: low RB leads to high p53
So p53 must be inactivated in cancer with Rb mutations
so p53 and RB are often both mutated in cancers
What kinase pathway does DNA damage induce?
ATM, ATR, and CHK2 which phosphorylate p53 and prevents MDM2 binding and increases the the stability of p53
What is the correlation between Ataxia Telangiectasia patients and ATM
AT patients lack ATM so they are extremely sensitive to IR, develop multiple tumors early on, DO not delay DNA synthesis upon DNA damage by X-ray
so a lack in ATM yield similar results to p53 knockout, exhibit the same type of tumors
up to 1% of the population may be ATM ± and do not exhibit a phenotype unless they are exposed to X-rays
diagnostic rays can lead to
How does ATM sense DNA damage
Repair proteins bind ds breaks and other abnormal DNA structures and recruit PI3 kinases (ATM, ATR)
Other factors such as CHK2/1 and p53 are recruited and prophorylated
Leads to cell cycle arrest
How does caffeine imhibit checkpoints?
Enhances the toxicity of radiation
caffeine inhibits CHK1/2, ATM, ATR
Will not phosphorylate p53 making it susceptible to MDM2 binding and leads to degradation
p53 as a guardian gene
It responds to multiple signals that threaten the integrity of the genome and elicits multiple effects that protect the integrity
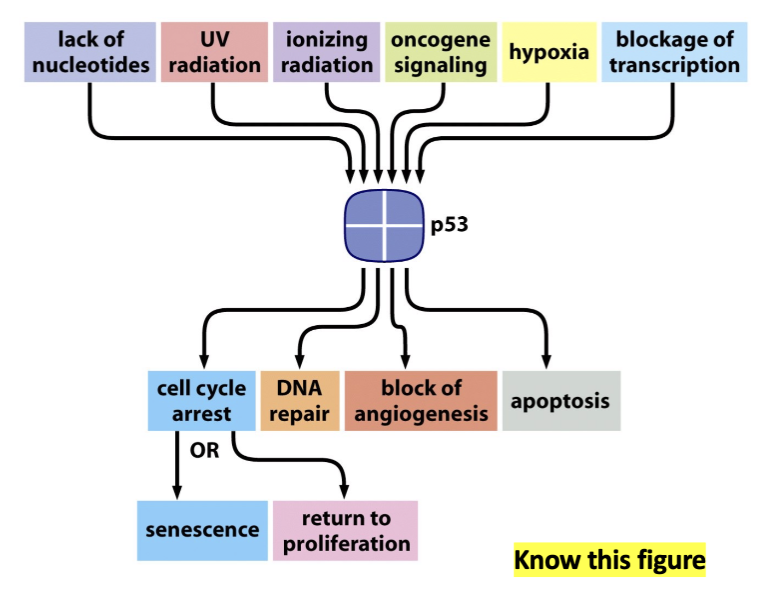
The role p53 plays in DNA pair
Binds XPD, XPB, CSB
increases translation of genes involved in DNA repair like GADD45 for growth arrest and DNA damage
decreases translesion synthesis by decreasing the translation of PCNA (proliferating cell nuclear antigen)
PCNA: increases the processicity of error-prone DNA polymerase
So more wt p53 → less PCNA → fewer mutations → genome guarded