Organic CHM 265 (Exam Two) - Purdue University
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
What is mass spectrometry?
An analytical technique that uses mass-to-charge ratios and the abundance of gas-phase ions to identify the amount and type of chemicals in a sample
What are the three main points of mass spectrometry?
1. The mass spectrum (relative = x) (mass-to-charge = y)
2. The base peak (the highest peak)
3. The relative abundance
What is the high-resolution mass of 1^H
1.00783 amu
What is the high-resolution mass of 2^H
2.01410 amu
What is the high-resolution mass of 12^C
12.0000 amu
What is the high-resolution mass of 13^C
13.0034 amu
What is the high-resolution mass of 14^N
14.0031 amu
What is the high-resolution mass of 15^N
15.0001 amu
What is the high-resolution mass of 16^O
15.9949 amu
What is the high-resolution mass of 18^O
17.9992
What is the high-resolution mass of 35^Cl
34.9689 amu (This is a halogen with a 3:1 ratio)
What is the high-resolution mass of 37^Cl
36.9659 amu (This is a halogen with a 3:1 ratio)
What is the high-resolution mass of 79^Br
78.9183 amu (This is a halogen with a 1:1 ratio)
What is the high-resolution mass of 81^Br
80.9163 amu (This is a halogen with a 1:1 ratio)
Which two elements have significant M+2 peaks?
Cl and Br (Halogens)
During a fragmentation, only what is detected by MS?
Cations (the radicals cannot be seen)
What is the Nitrogen Rule?
-An odd molecular weight indicates an odd number of nitrogen atoms
-An even molecular weight indicates the absence of nitrogen or an even number of nitrogen atoms
Where do fragmentations occur for Alkanes?
In the middle of the unbranched chains
Alkenes show what type of peak for MS?
They show a strong molecular ion peak - and form resonance-stabilized allylic cations
What is the most common fragmentations for alcohols?
The loss of H2O and the loss of an alkyl group
What is the pKa value for H2SO4?
-10 (mineral acid)
What is the pKa value for HCL?
-8 (mineral acid)
What is the pKa value for CF3CO2H
0
What is the pKa value for CH3CO2H?
5
What is the pKa value for NH4^+
9
What is the pKa value for Phenol?
10
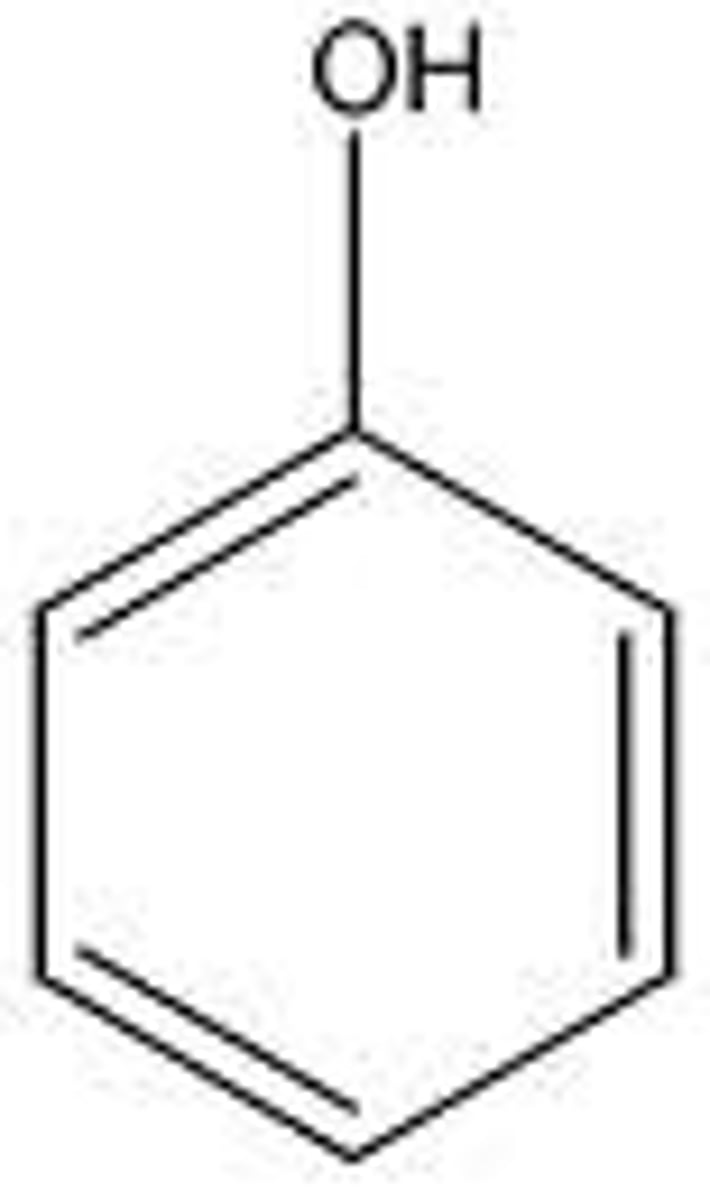
What is the pKa value for H2O
14
What is the pKa value for R-O-H
16-18
What is the pKa value for R-C≡CH
20-25
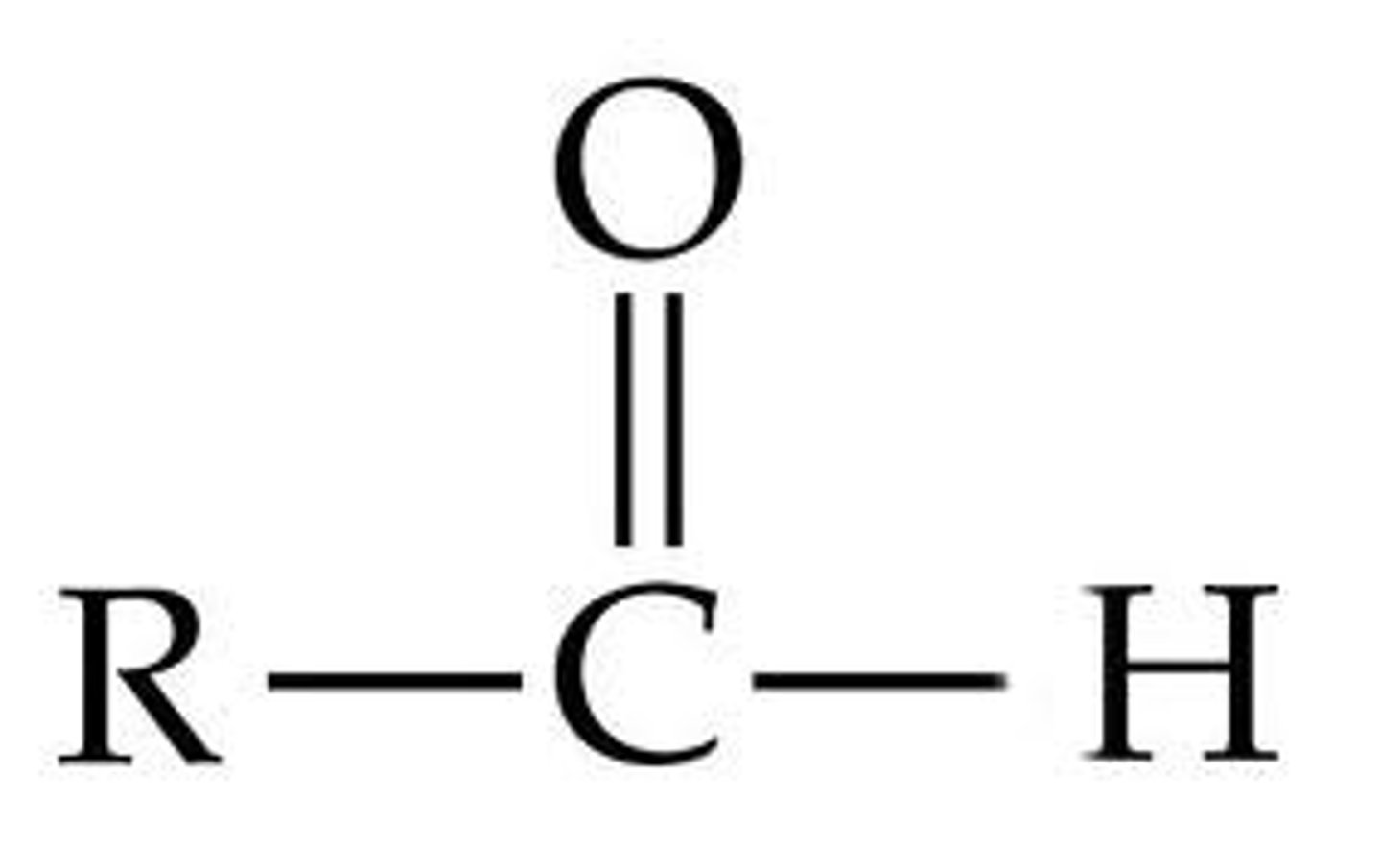
What is the pKa value for Aldehydes?
18-22
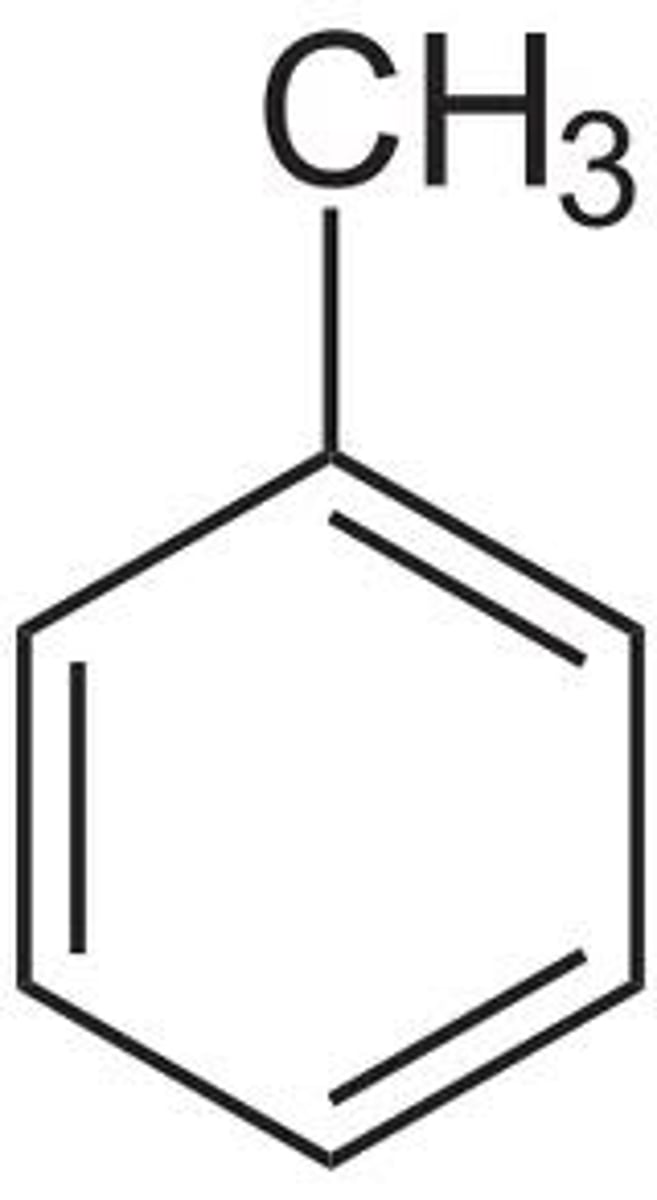
What is the pKa value for toluene 𝐂𝟔𝐇𝟓𝐂𝐇𝟑
35
What is the pKa value for R2N-H
R2N-H = 35
What is the pKa value for R3C-H
45-55
MS for Alkynes form what type of resonance-stabilized cation?
Propargyl Cations
α-cleavage is what?
A fragmentation process in mass spectrometry where a carbon-carbon bond adjacent to a functional group is broken, this is mainly in carbonyl groups.
McLafferty Rearrangment is what?
A fragmentation reaction in mass spectrometry where a carbonyl-containing molecule transfers a gamma-hydrogen to the carbonyl oxygen
A common loss of 15 in MS is considered what?
A loss of CH3
A common loss of 18 in MS is considered what?
A loss of H2O, (indicates alcohol, especially if the m/z 18 is observed)
A common loss of 28 in MS is considered what?
A loss of CH2≡CH2, CO (Cyclohexanes and aldehydes)
A common loss of 29 in MS is considered what?
A loss of C2H5, HCO, or H2CNH
A common loss of 14n+15 in MS is considered what?
A loss of alkyl [(CH2)nCH3)] = 15, 29, 43, 57, and 71
A common loss of 14n+17 in MS is considered what?
A loss of an alkoxy group [CnH2n+1O] --> Contains an O, and the peaks will be seen at 31, 45, 59, and 73