4.5.1.2 - Reaction profiles
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
when do chemical reactions occur?
chemical reactions can only occur when reacting particles collide with each other and with sufficient energy
what is activation energy (Ea)?
the minimum amount of energy that particles must have to react (to collide with each other)
So the greater the activation energy, the more energy needed to start the reaction
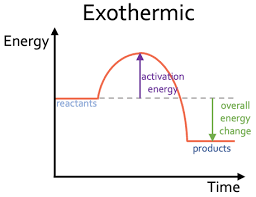
describe the reaction profile for an exothermic reaction.
as the products are at a lower energy than the reactants
difference in height = overall energy change in the reaction per mole
initial rise = activation energy
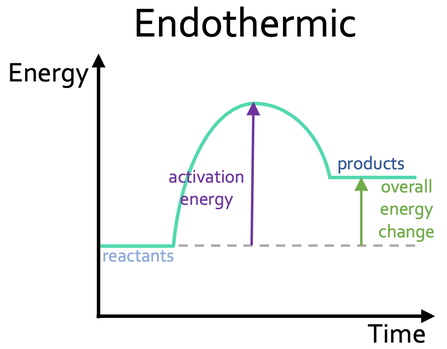
describe the reaction profile for an endothermic reaction.
products are at a higher energy than the reactants
difference in height = energy taken in per mole