P3 - Internal energy
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
What is specific heat capacity?
Energy needed to change the temperature of 1kg of a substance by 1°C
What is the equation that links specific heat capacity?
mass (kg) x specific heat capacity (J/kg°C) x temperature change (°C) = change in energy (J)
What is internal energy?
Total kinetic energy and potential energy of all the particles in a system
What is the equation of internal energy?
Kinetic energy + Potential energy = Internal energy
What happens if the internal energy of a system is increased?
Increases the temperature of the particles (kinetic energy)
Changes the state by breaking forces between particles (potential energy)
What happens when the internal energy of a system is decreased?
Decreases the temperature of the particles (kinetic energy)
Changes state by increasing forces between particles (potential energy)
When the internal energy is lost, where does it end up?
In the surroundings
What is specific latent heat?
Energy needed to change the state of 1kg of a substance with no change in temperature
What is specific latent heat of fusion?
Energy needed to changed 1kg of a solid into a liquid with no change in temperature (also is liquid to solid)
What is specific latent heat of vaporisation?
Energy needed to changed 1kg of a liquid into a gas with no change in temperature (also is gas to liquid)
What is the equation of specific latent heat?
Mass (kg) x specific latent heat (J/kg) = energy (J)
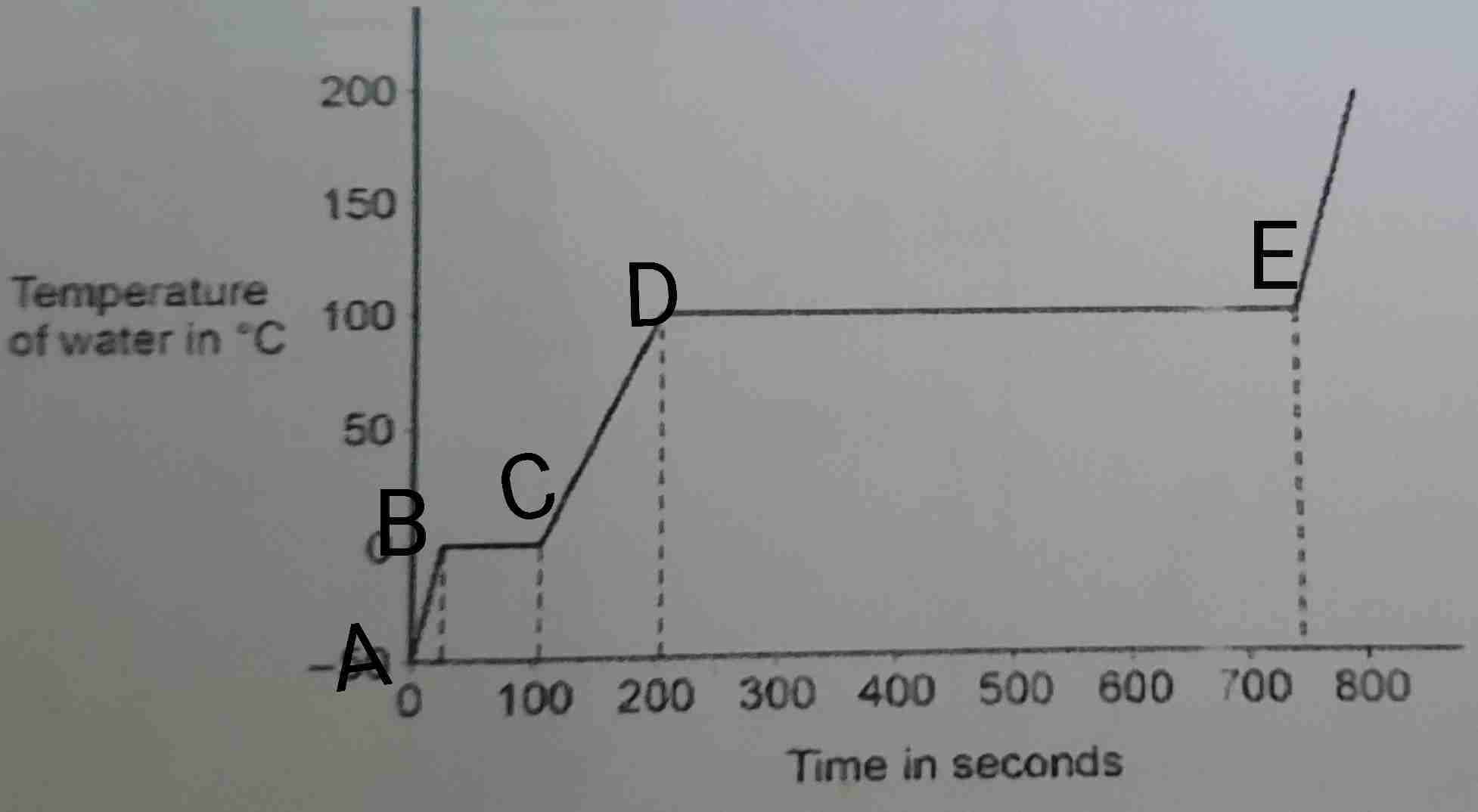
Looking at the heating curve graph for ice, how is the specific latent heat of vaporisation greater than the specific latent heat of fusion?
There is a much bigger flat line at points D to E rather than at points B to C. This shows that there is more time needed for boiling than melting
Why would the specific latent heat of vaporisation be greater than the specific latent heat of fusion?
A lot more energy is needed to break forces in a liquid rather than breaking forces in a solid
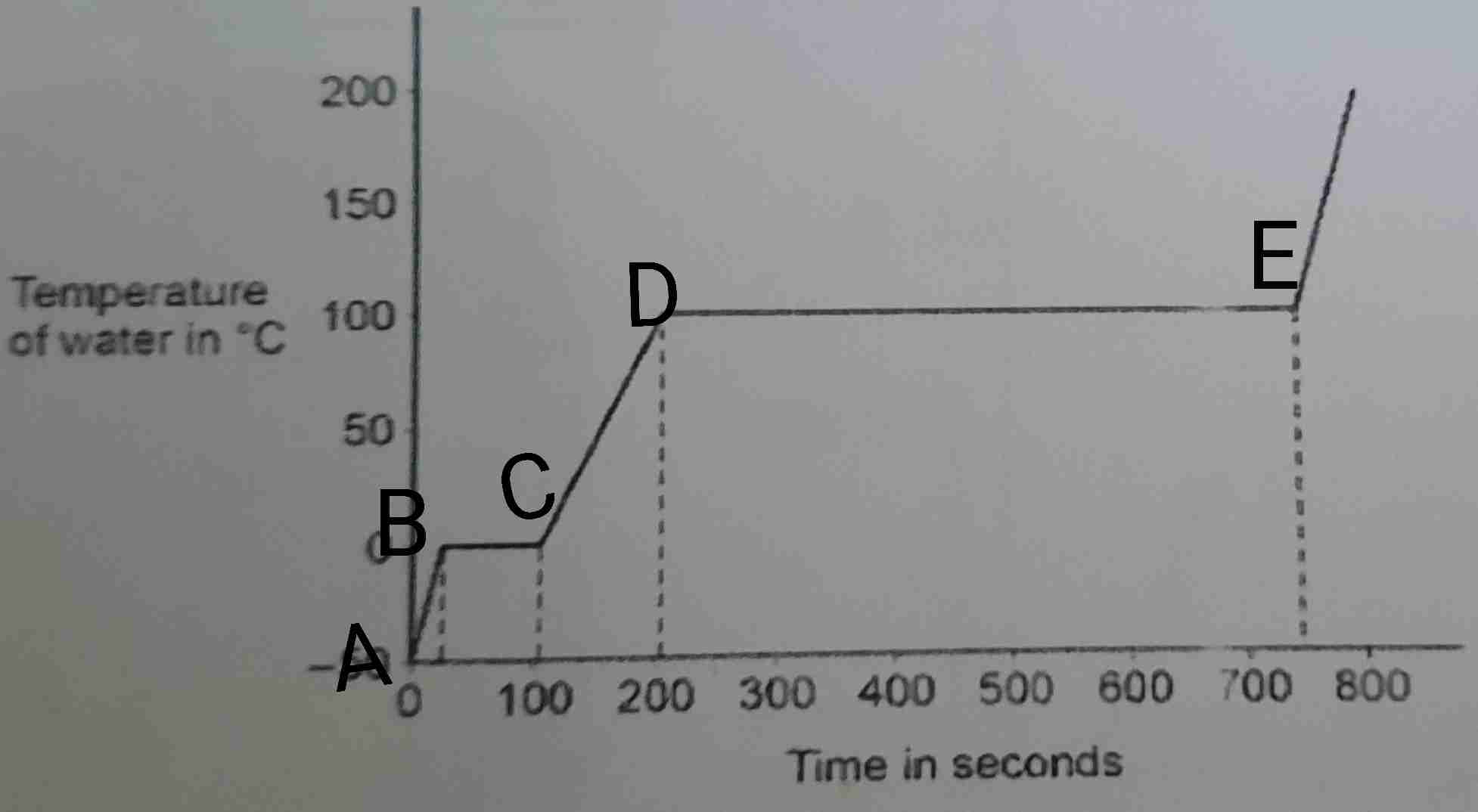
Looking at the heat curve graph for ice, how is the specific heat capacity of ice less than the specific heat capacity of a water?
The gradient for ice (0s) is steeper than the gradient for water (100s - 200s). This shows that ice has a lower specific heat capacity as it increases in temperature quicker and needs less energy to heat up
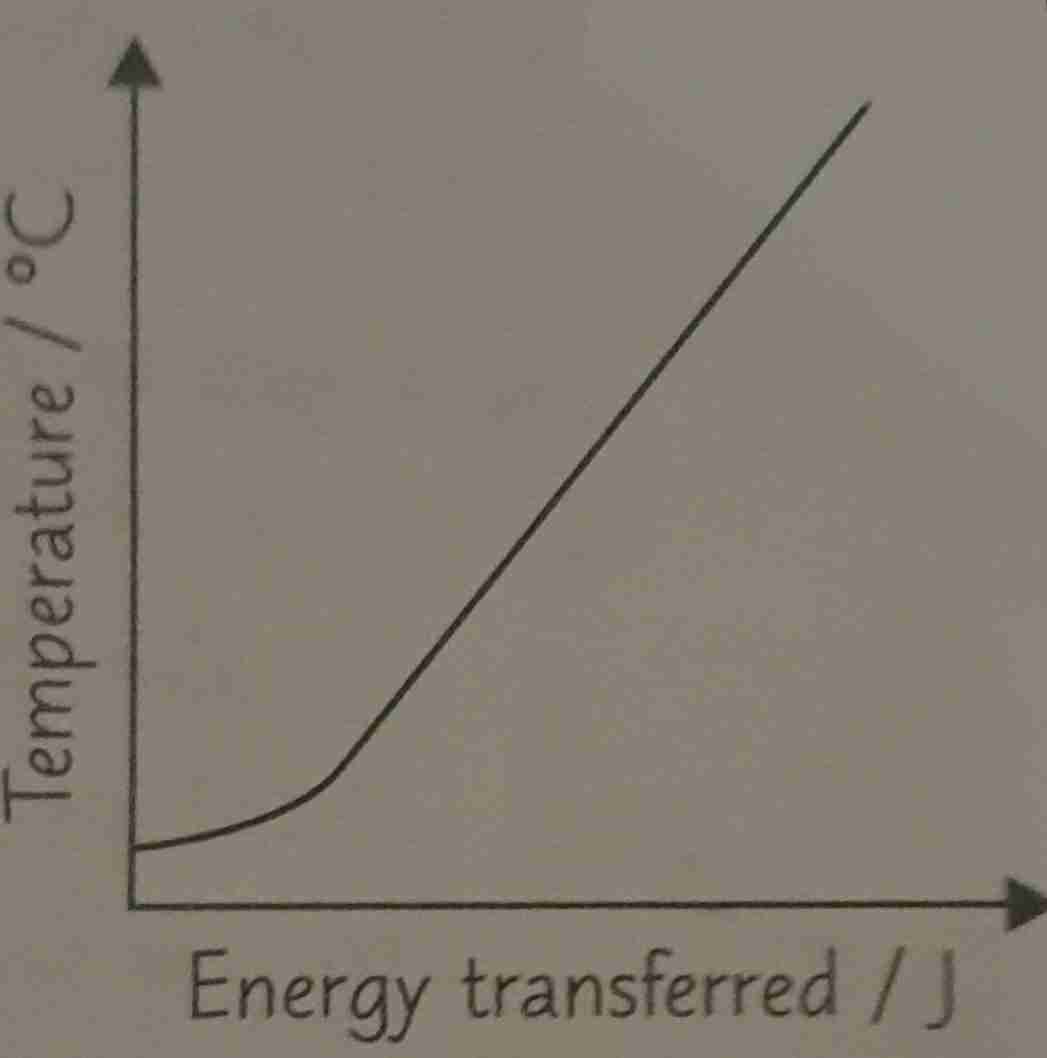
What errors could occur in the specific heat capacity practical?
Takes time for the heater to heat up - why graph is curved at start
Measured value of Specific heat capacity greater than actual value - heat energy lost to surroundings so temperature change not as high
Reduce second error by adding insulation
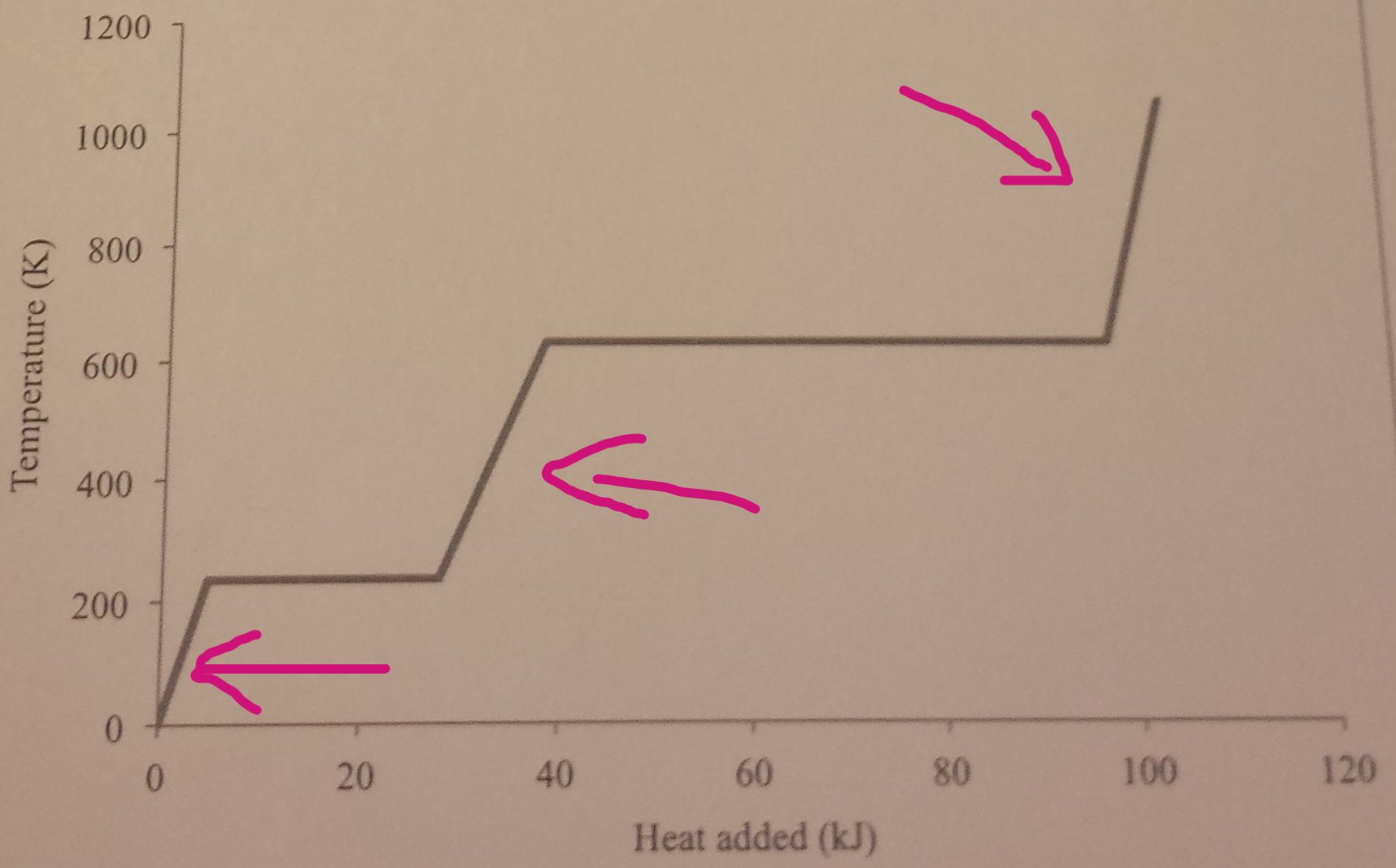
By looking at the graph, what is happening at the points where the arrows are pointing at?
A increase in internal energy increases the kinetic energy and temperature of particles
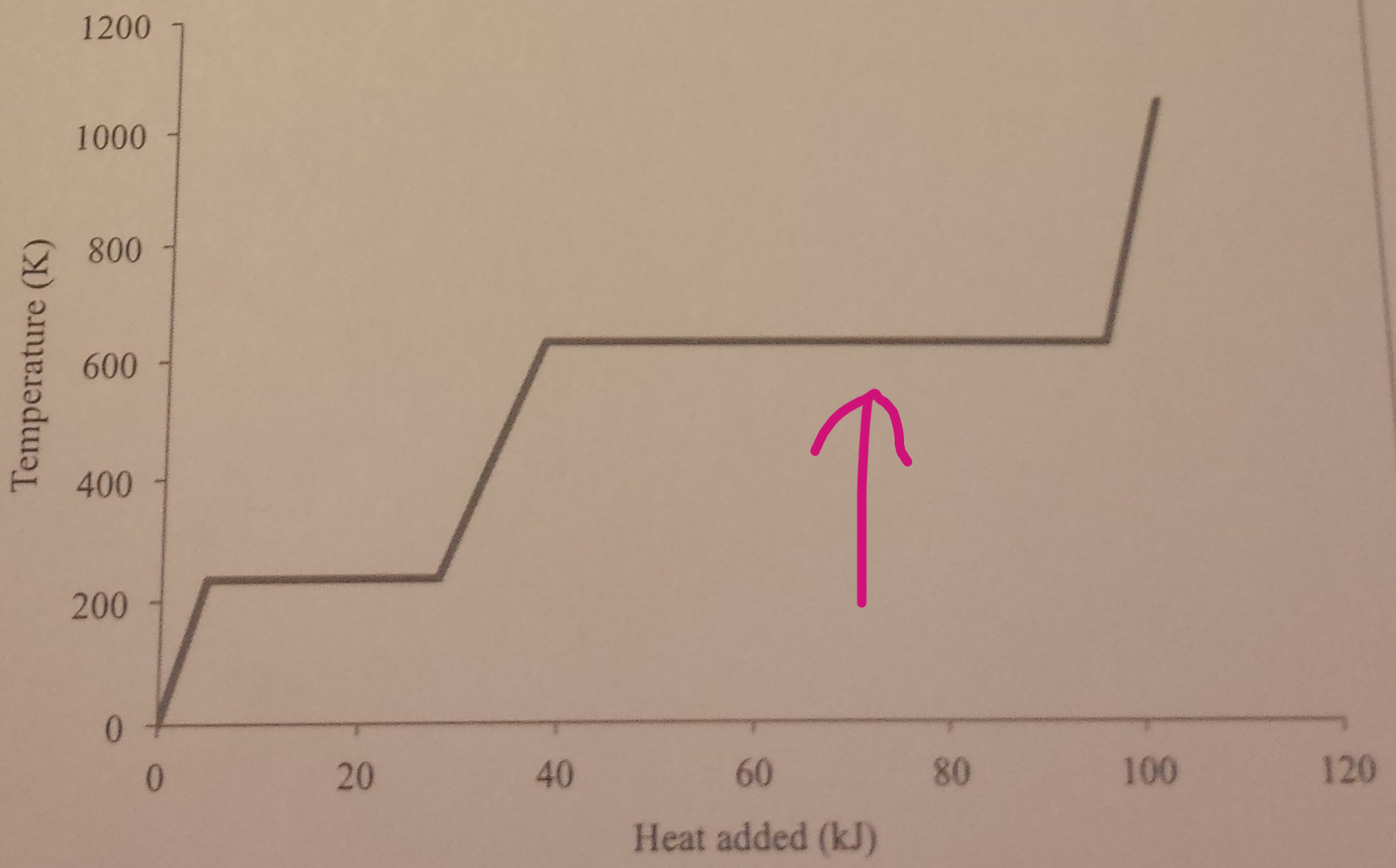
By looking at the graph, what is happening at the points where the arrows are pointing at?
When the liquid changes to a gas, there is no temperature change, as all the energy is being used to break forces of attraction. No change in kinetic energy, but increase in potential energy and internal energy
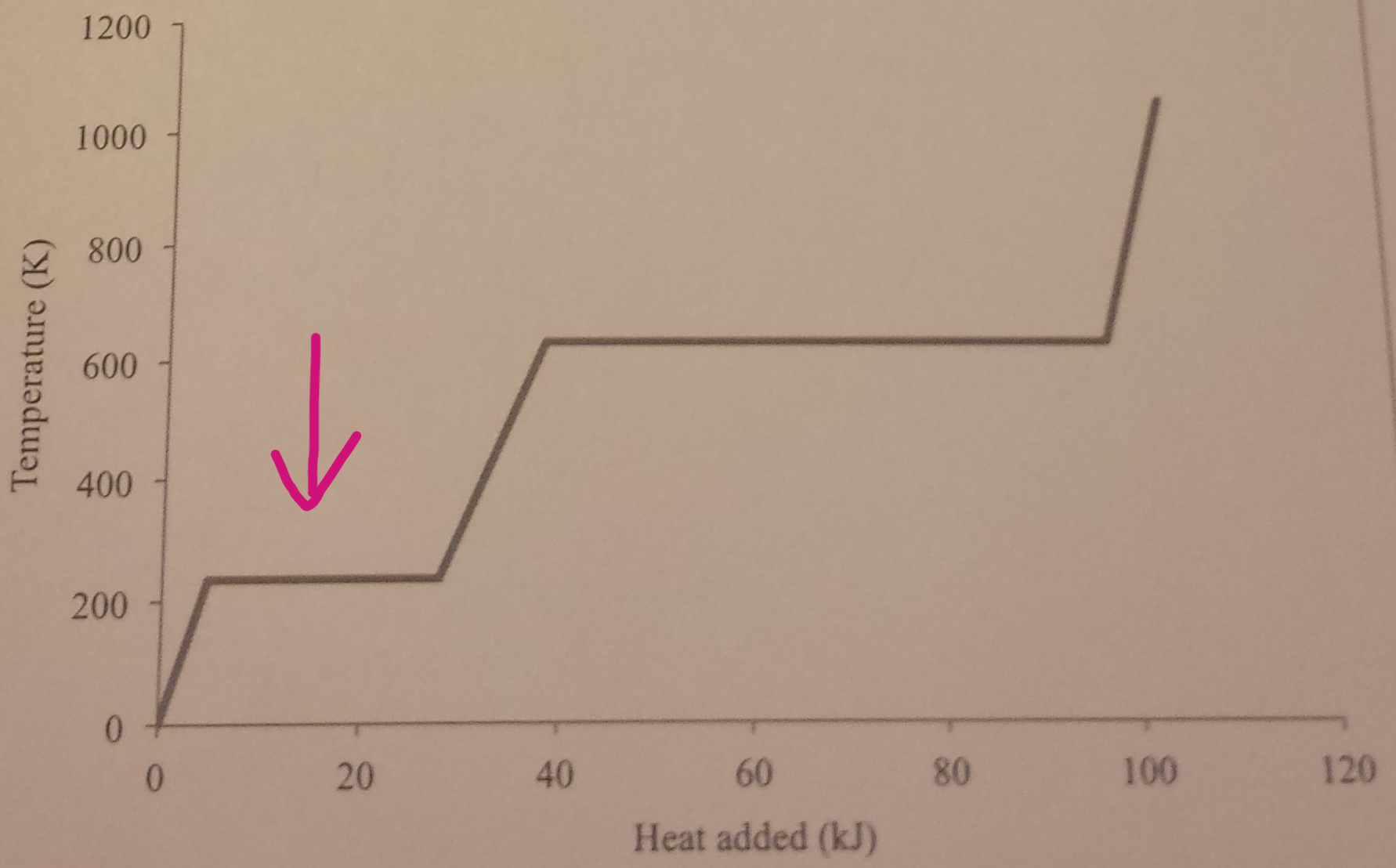
By looking at the graph, what is happening at the points where the arrows are pointing at?
When the solid changes into a liquid, there is no temperature change, as all the energy is being used to weaken forces of attraction. No change in kinetic energy, but increase in potential energy and internal energy

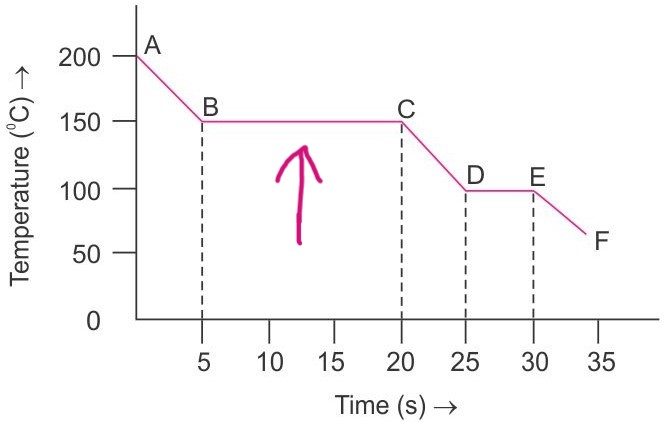
By looking at the graph, what is happening at the points where the arrows are pointing at?
A decrease in internal energy decreases the kinetic energy and temperature of particles
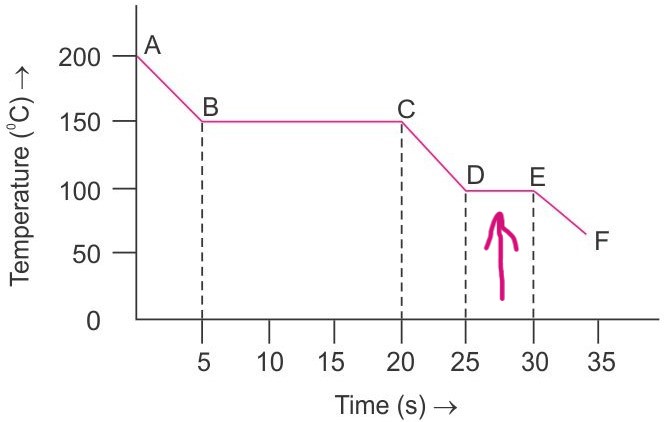
By looking at the graph, what is happening at the points where the arrows are pointing at?
When the gas changes to a liquid, there is no temperature change and energy is released to the surroundings. No change in kinetic energy, but decrease in potential energy and internal energy
By looking at the graph, what is happening at the points where the arrows are pointing at?
When the liquid changes into a solid, there is no temperature change and energy is released as stronger forces are made. No change in kinetic energy, but decrease in potential energy and internal energy
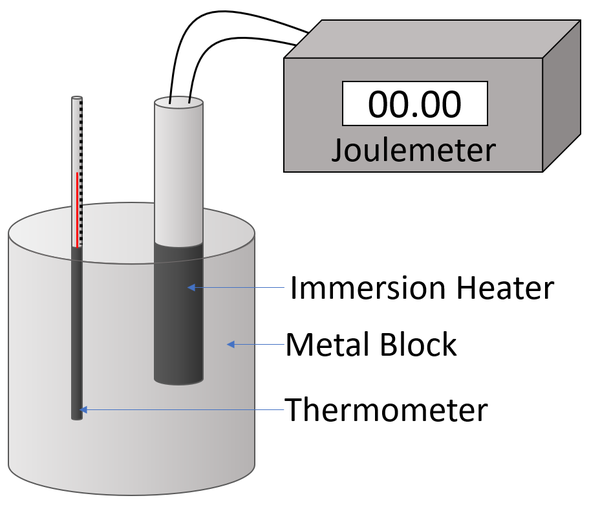
How to do the specific heat capacity practical?
Measure mass of metal block 2 holes in it, then insert a thermometer and immersion heater in the holes.
Connect the immersion heater to the power supply and the joulemeter and connect the joulemeter to the power supply
Measure start temperature of the block
Turn on the heater and measure the temperature of the block every min for 10 mins
Measure energy transferred using joulemeter
Calculate specific heat capacity
Plot results on graph