B1.1: Carbohydrates and Lipids | IB Biology HL
1/35
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
Outline the number and type of bonds carbon can form with other atoms.
B1.1.1: Chemical properties of a carbon atom allowing for the formation of diverse compounds on which life is based
Carbon can form up to 4 covalent bonds with other carbon bonds or other nonmetal elements.
The covalent bonds can be single bonds or a combination of single and double bonds.
Outline the cause and consequence of covalent bonds between atoms.
B1.1.1: Chemical properties of a carbon atom allowing for the formation of diverse compounds on which life is based
Covalent bonds occur when two atoms share one or more pair of valence electrons.
As a result, these atoms are bonded together.
List the four major classes of carbon compounds used by living organisms.
B1.1.1: Chemical properties of a carbon atom allowing for the formation of diverse compounds on which life is based
Carbohydrates
Lipids
Nucleic acids
Proteins
List example molecules with branched chain, unbranched chain, single ring or multiple rings.
B1.1.1: Chemical properties of a carbon atom allowing for the formation of diverse compounds on which life is based
Unbranched chains: fatty acids
Branched chains: triglycerides
Simple rings: glucose
Multiple rings: cholesterol
Define monomer and polymer.
B1.1.2: Production of macromolecules by condensation reactions that link monomers to form a polymer
A monomer is a subunit molecule that joins with other monomers to form polymers.
A polymer is a molecule made of subunits.
Describe condensation reactions.
B1.1.2: Production of macromolecules by condensation reactions that link monomers to form a polymer
In condensation reactions, two molecules combine by losing 2 hydrogen atoms and one oxygen atom. It releases H2O (water) as a byproduct.
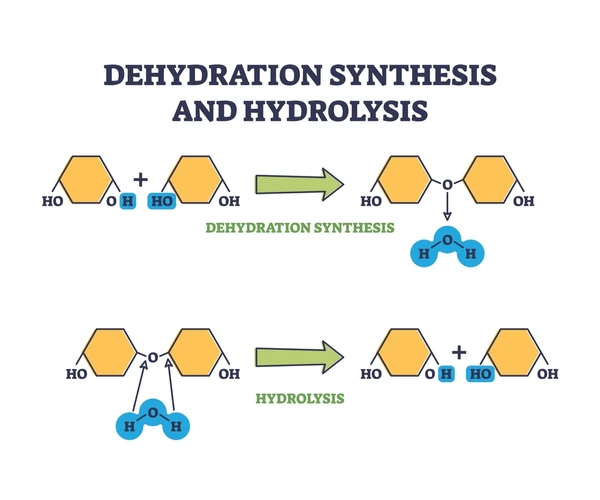
State that energy from ATP is needed to produce macromolecules by condensation reactions.
B1.1.2: Production of macromolecules by condensation reactions that link monomers to form a polymer
Energy from ATP is needed to produce macromolecules by condensation reactions.
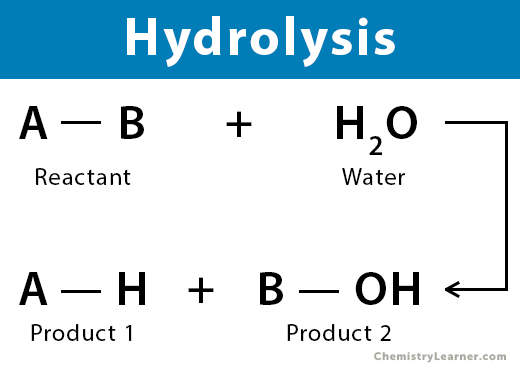
Describe hydrolysis reactions.
B1.1.3: Digestion of polymers into monomers by hydrolysis reactions
In hydrolysis reactions, a water molecule is added to break down a large molecule.
Define monosaccharide.
B1.1.4: Form of function of monosaccharides
Monosaccharides are a large group of organic compounds with one sugar molecule (the monomers of polysaccharides).
Identify pentose and hexose carbohydrates from molecular diagrams.
B1.1.4: Form of function of monosaccharides
A pentose sugar contains 5 carbons.
Hexose sugars contain 6 carbons.
Draw a diagram of ribose and deoxyribose.
B1.1.4: Form of function of monosaccharides
Outline the properties of glucose referring to solubility, transportability, stability, and energy yield from oxidation.
B1.1.4: Form of function of monosaccharides
Glucose is a monosaccharide and has the following properties:
Soluble in water: Glucose is a polar molecule which readily dissolves in water
Transportability: Since glucose is soluble in water, it is transported within body fluids (like bloodstreams in humans)
Chemical stability: Glucose is a relatively stable compound, so it does not degrade as it is being transported
Energy yield: Glucose is the primary fuel for respiration in cells
Glucose molecules are repeatedly oxidized to produce a net gain of up to 36 ATP molecules.
Define polysaccharide.
B1.1.5: Polysaccharides as energy storage compounds.
A polysaccharide is a long chain of carbohydrate molecules made of smaller monosaccharides.
Compare the structure and function of amylose, amylopectin, and glycogen.
B1.1.5: Polysaccharides as energy storage compounds.
Amylose and amylopectin are types of starches. Amylose, amylopectin, and glycogen are all made of long chains of alpha-glucose molecules. Their glucose molecules are all bonded with glycosidic bonds between carbon-1 and carbon-4.
Amylose is simply a straight chain polymer. Amylopectin is a branched chain polymer where every 20th glucose molecule adds an additional glucose molecule bond at carbon-6. Glycogen is made of chains of many branches of alpha glucose with alpha glucose bonding to carbon-6, with more branches than amylopectin.
Amylose and amylopectin are both large molecules that are not very soluble in water (some may be soluble in hot water). Glycogen has many branches and coiling, so it is an insoluble molecule.
Starches (amylose and amylopectin) are used to compactly store starch grains in plant cells without impacting osmotic pressure in cells, so they are used to store energy in plants. Glycogen is used for short-term energy storage in animals.
Discuss the benefit of polysaccharide coiling and branching during polymerization.
B1.1.5: Polysaccharides as energy storage compounds.
Polysaccharide coiling and branching during polymerization allows starch and glycogen to be compact. Ultimately, this allows for the storage of many glucose molecules.
Explain how condensation or hydrolysis of alpha-glucose monomers build or mobilize energy stores.
B1.1.5: Polysaccharides as energy storage compounds.
The condensation of alpha-glucose monomers allows glucose molecules to be added onto amylose, amylopectin, and glycogen. Thus, when there is extra energy (glucose molecules), it can easily be stored.
The hydrolysis of alpha-glucose monomers allows glucose molecules to be removed from amylose, amylopectin, and glycogen. As a result, when energy needs to be used, a hydrolysis reaction can be used to release glucose molecules from the energy store.
Compare the structure of alpha-glucose and beta-glucose.
B1.1.6: Structure of cellulose related to its function as a structural polysaccharide in plants
Alpha-glucose and beta-glucose are both (monosaccharide) hexose sugars. Their carbons are labelled 1 to 6 clockwise after the Oxygen. Both typically form bonds between carbon-1 and carbon-4 (during the formation of polysaccharides).
The orientation of the hydroxyl group differs between alpha-glucose and beta-glucose. Alpha-glucose has both hydroxyl groups on the same side. Beta-glucose has hydroxyl groups on opposite sides.
Describe the structure of cellulose microfibrils.
B1.1.6: Structure of cellulose related to its function as a structural polysaccharide in plants
Cellulose is an unbranched polysaccharide composed of beta glucose molecules found in the cell wall of plants.
Cellulose molecules have long chains of beta glucose molecules bonded between carbon-1 and carbon-4. Every second beta glucose molecule is flipped, which creates a straight chain of cellulose molecules
Microfibrils are groups of cellulose molecules held together by hydrogen bonds.
Discuss the consequences of the strength of cellulose in the plant cell wall.
B1.1.6: Structure of cellulose related to its function as a structural polysaccharide in plants
Cellulose microfibrils have high tensile strength. Therefore, they can maintain the structural integrity of cell walls in plants.
Outline an example of a function of a glycoprotein.
B1.1.7: Role of glycoproteins in cell-cell recognition
Glycoproteins are integral proteins located within teh phospholipid bilayers of cells.
Glycoproteins have many functions:
Cell to Cell Adhesion: Interacts with glycoproteins on neighboring cells, allowing the formation of tissues
Receptors: Glycoproteins act as receptors for hormones
When a hormone binds to a specific glycoprotein receptor, it changes metabolism within the cell
Cell to Cell Communication: Neurotransmitters bind to glycoproteins, allowing communication between cells
Immune Response: Glycoproteins act as markers on cells allowing the immune system to distinguish between self and non-self cells
Compare the structure of the A, B and O glycoproteins on the red blood cell membrane.
B1.1.7: Role of glycoproteins in cell-cell recognition
Glycoproteins act as antigens (substances that stimulate an immune response and the production of antibodies) in red blood cells. If the glycoprotein is not recognized by the immune system, it will be attacked.
Erythrocytes (RBC) can have antigen A or antigen B. Blood Group A has antigen A on the RBC membrane. Blood Group B has antigen B on the RBC membrane. Blood group O doesn’t have any antigens on the RBC membrane.
Discuss the consequences of the presence of A, B and O glycoproteins during blood transfusion.
B1.1.7: Role of glycoproteins in cell-cell recognition
The presence of A, B, and O glycoproteins during blood transfusion is very consequential. If the blood type does not match the receiver’s blood type, the immune system will attack those red blood cells because it does not recognize the antigen.
A donor with Type A blood type can only give blood to recipients with Type A or AB blood types. A donor with Type B blood type can only give blood to recipients with Type B or Type AB blood types. A donor with Type AB blood type can only give blood to recipients with Type AB blood type. Otherwise, the recipient’s immune system will attack the transfused RBC. A donor with Type O blood type is considered a “universal donor” because they can give blood to anyone (not considering Rh factor). A donor with -Rh blood type can give blood to recipients who have +Rh type, but +Rh can only give to +Rh.
A recipient with Type A blood type can only receive Type A or Type O blood. A recipient with Type B blood type can only receive Type B or Type O blood. A recipient with Type AB blood type is a “universal receiver” because they can receive blood from any blood type. A recipient with Type O blood type can only receive Type O blood. A donor with -Rh blood type can only receive -Rh blood, but a person with +Rh blood can receive both +Rh and -Rh.
Explain why lipids are hydrophobic.
B1.1.8: Hydrophobic properties of lipids
Lipids are substances in living organisms that dissolve in nonpolar solvents but aren’t commonly soluble in aqueous solutions.
Lipids contain many areas of hydrocarbons (just containing hydrogen and carbon). There is a nonpolar covalent bond between carbon and hydrogen, so lipids are mostly hydrophobic. They have low solubility in aqueous solutions but can dissolve quite well in nonpolar solvents.
Outline the structure and function of fats, oils, waxes and steroids.
B1.1.8: Hydrophobic properties of lipids
Fats are a type of triglyceride (esters composed of 3 fatty acid units linked to glycerol) made of saturated fatty acids. Fats function as a long-term energy source in animals.
Oils are a type of triglyceride (esters composed of 3 fatty acid units linked to glycerol) made of unsaturated fatty acids. Oils function as a long-term energy source in plants.
Waxes are made of long-chain fatty acid linked through an ester oxygen to a long-chain alcohol. Waxes function as a protective layer against water loss in plant leaves and animal skin.
Steroids have 4 rings of carbon atoms. Steroids function as hormones in plants an animals and is a structural component of animal cell membranes.
Explain the condensation reaction connecting fatty acids and glycerol to form a triglyceride.
B1.1.9: Formation of triglycerides and phospholipids by condensation reactions
Formation of triglycerides (connecting fatty acids): 1 glycerol (3-carbon molecule) + 3 fatty acids —> 1 triglyceride + 3 water molecules
Glycerol is a 3-carbon molecule. Each carbon bonds to 1 hydroxyl group initially
First, the OH group on the carboxyl group of fatty acid #1 (R1) aligns with glycerol’s OH group. A condesnation reaction occurs, releasing 1 H2O.
The condensation reaction forms a covalent bond between fatty acid #1 (R1) and the glycerol molecule.
A second fatty acid (R2) aligns with the 2nd OH group. A second condensation reaction occurs, which releases a second water molecule.
The second condensation reaction creates a new covalent bond between R2 and the glycerol molecule.
A third fatty acid (R3) aligns with the 3rd OH group. A third condensation reaction occurs, which releases a third water molecule.
The third condensation reaction creates a new covalent bond between R3 and the glycerol molecule. This forms a triglyceride.
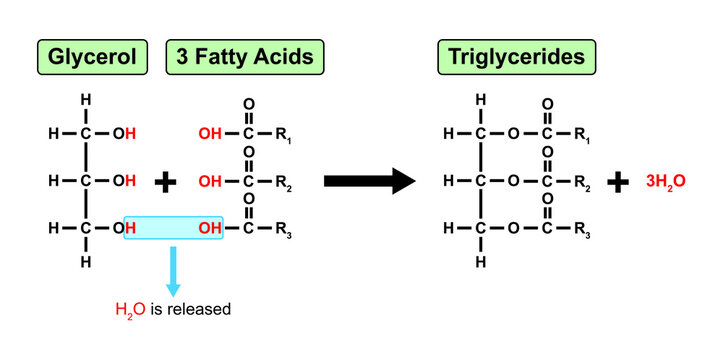
Explain the condensation reaction connecting fatty acids, glycerol and a phosphate group to form a phospholipid.
B1.1.9: Formation of triglycerides and phospholipids by condensation reactions
Formation of phospholipid (connecting fatty acids, glycerol, phosphate group): 1 glycerol + 2 fatty acids + 1 inorganic phosphate —> 1 phospholipid + 3 water molecules.
First, the OH group on the carboxyl group of fatty acid #1 (R1) aligns with glycerol’s OH group. A condesnation reaction occurs, releasing 1 H2O.
The condensation reaction forms a covalent bond between fatty acid #1 (R1) and the glycerol molecule.
A second fatty acid (R2) aligns with glycerol’s 2nd OH group. A second condensation reaction occurs, which releases a second water molecule.
The second condensation reaction creates a new covalent bond between R2 and the glycerol molecule.
A phosphate group aligns with glycerol’s 3rd OH group. A third condensation reaction occurs, which releases a third water molecule.
The condensation reaction forms a covalent bond between the phosphate group and the glycerol. This forms a phospholipid.
Describe the structure of a generalized fatty acid.
B1.1.10: Difference between saturated, monounsaturated, and polyunsaturated fatty acids.
A generalized fatty acid has a hydrophilic head and two hydrophobic tails.
The hydrophilic head is made of a phosphate molecule. A glycerol backbone connects the head to the two hydrophobic tails made of hydrocarbons. One tail is made of a saturated fatty acid, the other is unsaturated.
Compare and contrast the structures and properties of saturated and unsaturated (mono- or poly-) fatty acids.
B1.1.10: Difference between saturated, monounsaturated, and polyunsaturated fatty acids.
Saturated fatty acids only contains single bonds between carbons. All carbon bonds, except to the carboxyl group, are bonded to hydrogens (the molecule is “saturated” with hydrogens). Monounsaturated fatty acids have one double bond between 2 of the carbons in the hydrocarbon chain of the molecule, while Polyunsaturated fatty acids have more than one double bond between carbons.
Saturated fatty acids have a relatively high melting point and are solid (fat) at room temperature. Monounsaturated fatty acids have a lower melting point than saturated fatty acids and are liquid (oil) at room temperature. Polyunsaturated fatty acids have a relatively low melting point and are liquid (oil) at room temperature. Triglycerides with only saturated fatty acids are fats and are typically used by animals to store excessive energy. Monounsaturated fatty acids are used by some animals and many plants to store excess energy. Polyunsaturated fats are used by many plants to store energy.
Distinguish between the structure and properties of cis- and trans-unsaturated fatty acids.
B1.1.10: Difference between saturated, monounsaturated, and polyunsaturated fatty acids.
Cis- configuration has 2 hydrogens associated with the bond on the same side. Trans- configuration has 2 hydrogens associated with the bond on opposite sides.
Cis- configuration generally generates a kink/bend in the fatty acid. Trans- configuration usually allows the fatty acid to remain straight.
Cis- usually has a low melting point; some are liquid at room temperature). Trans- configuration usually has a high melting point and are solid at room temperature.
Cis- fatty acids occur naturally and are generally good for health. Trans- fatty acids are usually from processed foods; they lower levels of good cholesterol and increase the level of bad cholesterol.
Outline properties of triglycerides that make them suitable for long-term energy storage.
B1.1.11: Triglycerides in adipose tissues for energy storage and thermal insulation
Triglycerides are insoluble in body fluids. Therefore, they will not move from their storage sites. As a result, they are useful for long-term energy storage.
In addition, triglycerides can undergo hydrolysis reactions to produce glycerol and fatty acids that are then available for energy in cellular respiration.
State the function of adipose tissue.
B1.1.11: Triglycerides in adipose tissues for energy storage and thermal insulation
Adipose tissue stores fat (excess energy) in the form of triglycerides.
Discuss the adaptation of a thick adipose tissue layer as a thermal insulator.
B1.1.11: Triglycerides in adipose tissues for energy storage and thermal insulation
A thick layer of adipose tissue is common in organisms living in cold regions (like the arctin).
Endotherms are organisms that maintain steady internal temperature regardless of their environmental temperature. A thick adipose tissue (blubber) found between skin and muscles helps trap the heat generated by the inner metabolic activities of the animal.
Draw a simplified diagram of the structure of the phospholipid, including a phosphate-glycerol head and two fatty acid tails.
B1.1.12: Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
Define hydrophilic, hydrophobic and amphipathic.
B1.1.12: Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
Hydrophilic means “water-loving.” It refers to polar or ionic molecules that are capable of forming hydrogen bonds with water molecules. They attract water molecules and are soluble in water.
Hydrophobic means “water-fearing.” It refers to nonpolar molecules that are incapable of forming bonds with water molecules. They repel water molecules are are insoluble in water (but may be soluble in nonpolar solvents).
Amphipathic molecules are a single molecule that has both hydrophilic and hydrophobic regions. Amphipathic molecules will spontaneously arrange themselves with hydrophilic regions towards water& hydrophobic regions away from water.
Outline the amphipathic properties of a phospholipid.
B1.1.12: Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
A phospholipid has 2 hydrophobic fatty acid tails and a polar phosphate group head.
Explain why phospholipids form bilayers in water, with reference to hydrophilic phosphate heads and two hydrophobic hydrocarbon tails.
B1.1.12: Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic regions
Phospholipids naturally form a bilayer in water because they are amphipathic molecules.
A phospholipid has 2 hydrophobic fatty acid tails extending towards each other, so they repel water and want to keep away from the aqueous solution. Therefore, it will be on the “inside” of the bilayer to avoid the aqueous solution.
A phospholipid also has a polar phosphate group that is attracted to the aqueous solution.
Therefore, the phospholipids will naturally form a bilayer, with the inside being the hydrophobic tails that make up the cytoplasm, and the outside being the polar phosphate group heads touching extracellular fluid. This forms the plasma membrane.