11 - pentose phosphate pathway
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
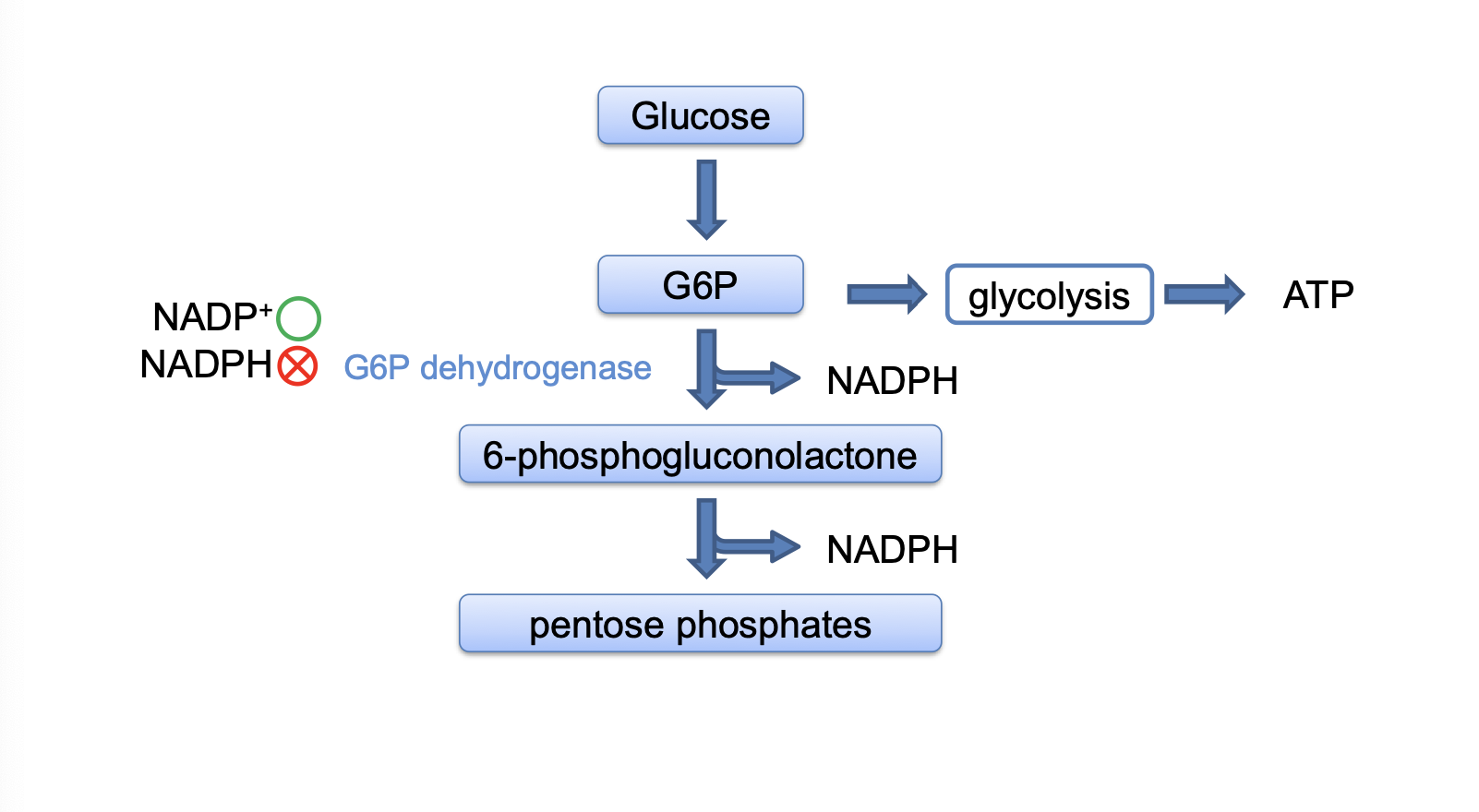
oxidative steps enzymes
G6P dehydrogenase
6-phosphogluconolactonase
6-phosphonogluconate dehydrogenase
purpose of the oxidative phase
oxidize glucose to ribulose-5-phosphate and make NADPH
non oxidative steps enzymes
ribulose-5-phosphate isomerase
ribulose-5-phosphate epimerase
transketolase
transaldolase
purpose of the non-oxidative phase
makes ribose which is needed to make DNA and RNA
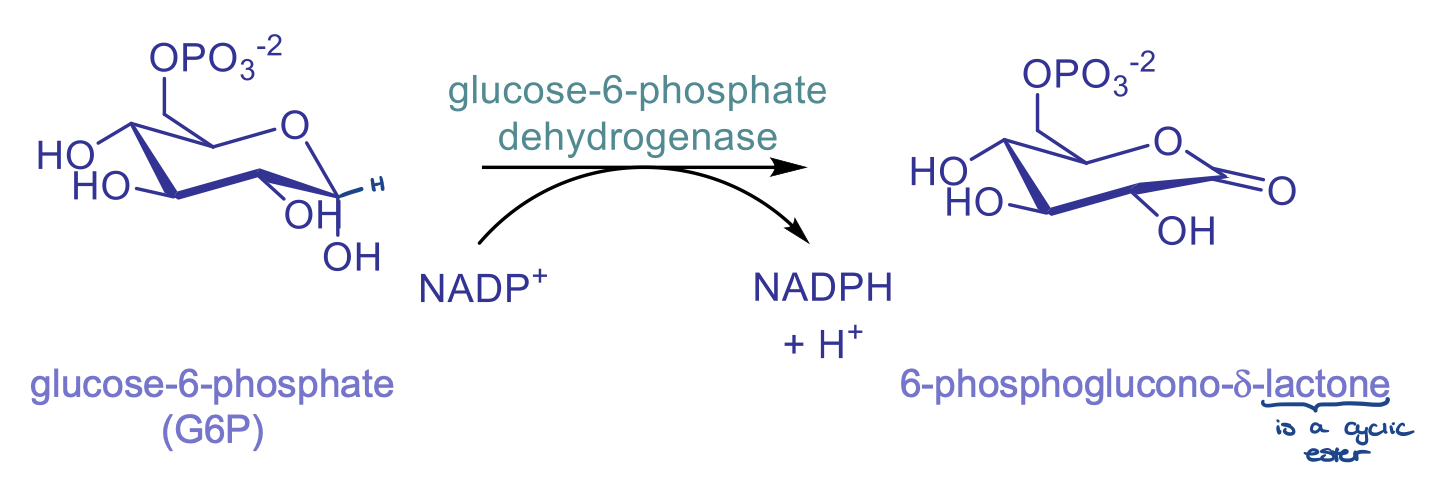
rxn 1: dehydration of g6p
substrate
enzyme
product
consequence of this reaction
catalyzed by G6P dehydrogenase, involves reduction of NADP+ to NADPH
product: 6-phosphoglucono-d-lactone is a cyclic ester
committed step
once glycose undergoes this step it has to keep going, cannot go backwards in rxn
therefore this step is highly regulated
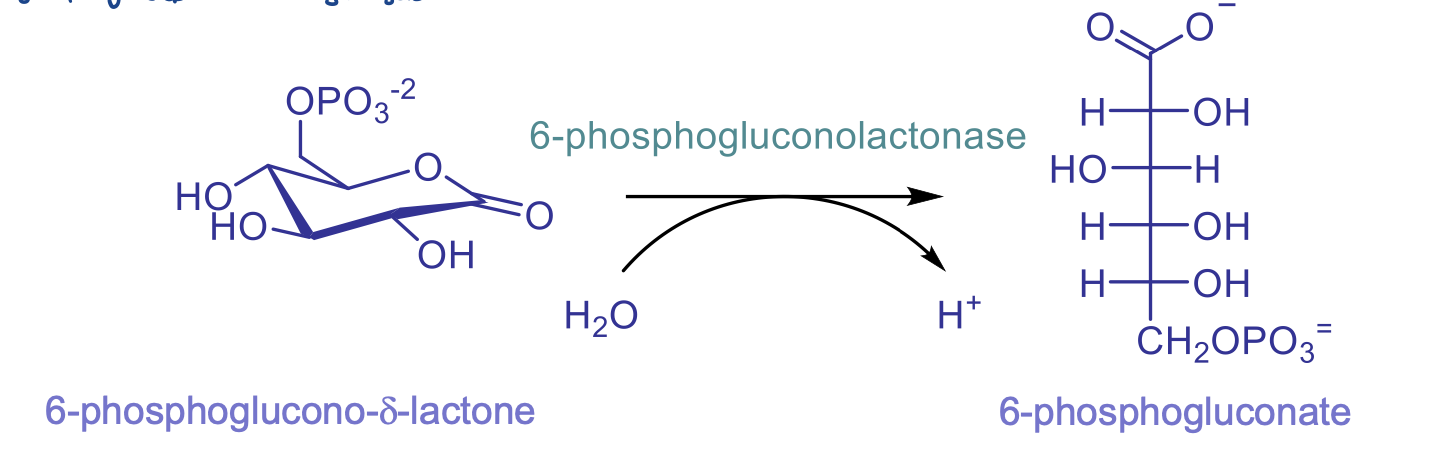
rxn 2: lactone hydrolysis
substrate
enzyme
product
overall, what happens in this reaction?
is the enzyme needed?
catalyzed by 6-phosphogluconolactonase and involves H2O to H+
ring-opening step
enzyme not acc needed for this step but helps to speed it up
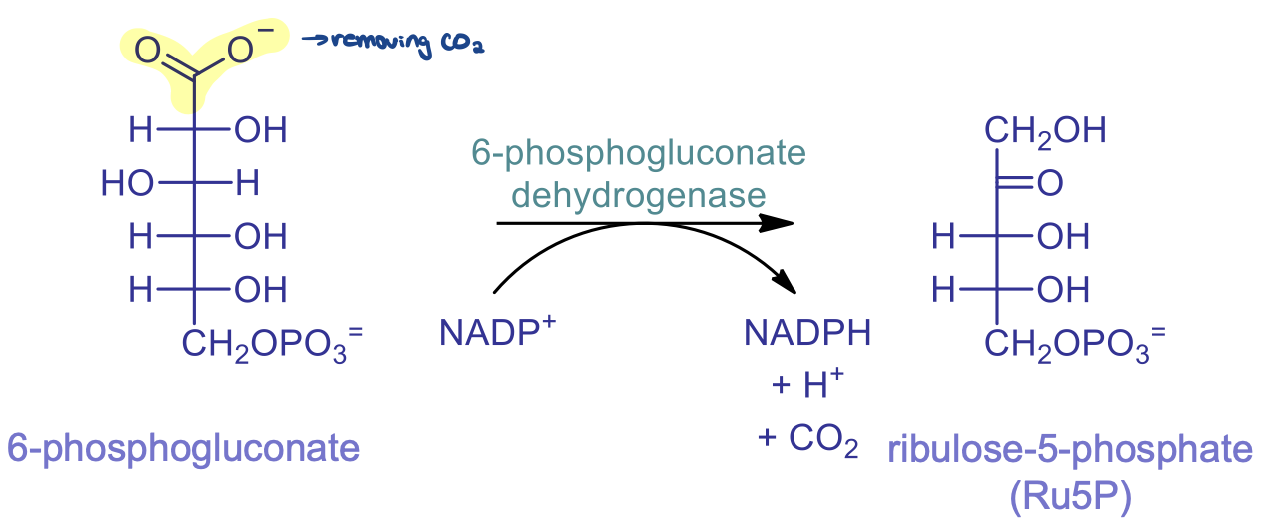
rxn 3: oxidative decarboxylation
substrate
enzyme
product
overall what happens?
catalyzed by 6-phosphonogluconate dehydrogenase and involves reduction of NADP+ to NADPH
2 part reaction: dehydrogenation (oxidation of CHO) and decarboxylation of Beta-keto acid (removal of CO2 from 6-phosphogluconate)
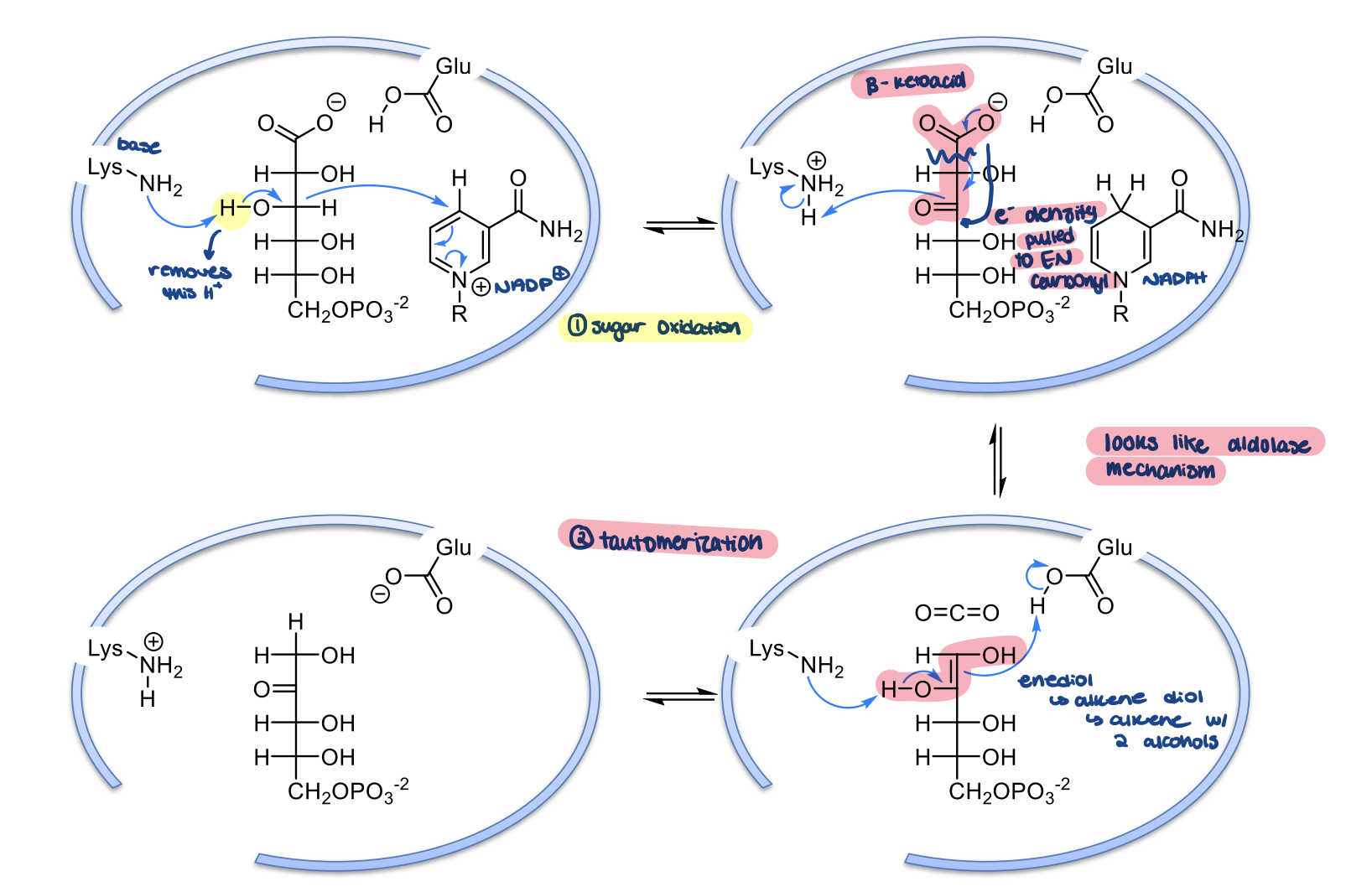
mechanism of 6-phosphonogluconate dehydrogenase
active site Lysine residue acts as a base to remove a H from carbon 3 of 6-phosphogluconate (gets oxidized to a carbonyl). Carbon 3 gives up its H so that the O can form its double bond/carbonyl. The H gets transferred to NADP+ to make NADPH
the B-keto acid undergoes resonance and dissociates as CO2. This causes electrons to shift to form a double bond between carbons 1 and 2. This causes carbonyl (carbon 2) to take back its H+ from Lysine to form OH group again. A enediol intermediate is formed. NADPH is released
The double bond between carbons 1 and 2 is used to pick up a H from a Glu residue in the active site. Lysine takes back the H on the OH group on carbon 2, so a carbonyl will form again. This is similar to an aldose mechanism
ribulose-5-phosphate (Ru5P) is formed
rxns 4: Ru5P isomerase
substrate
enzyme
product and what the product can be used for
overall, what occurs?
catalyzed by ribulose-5-phosphate isomerase
ribose-5-phosphate (R5P) is made which can continue in pentose phosphate pathway or be used to make DNA and RNA
switch the carbon the carbonyl is on
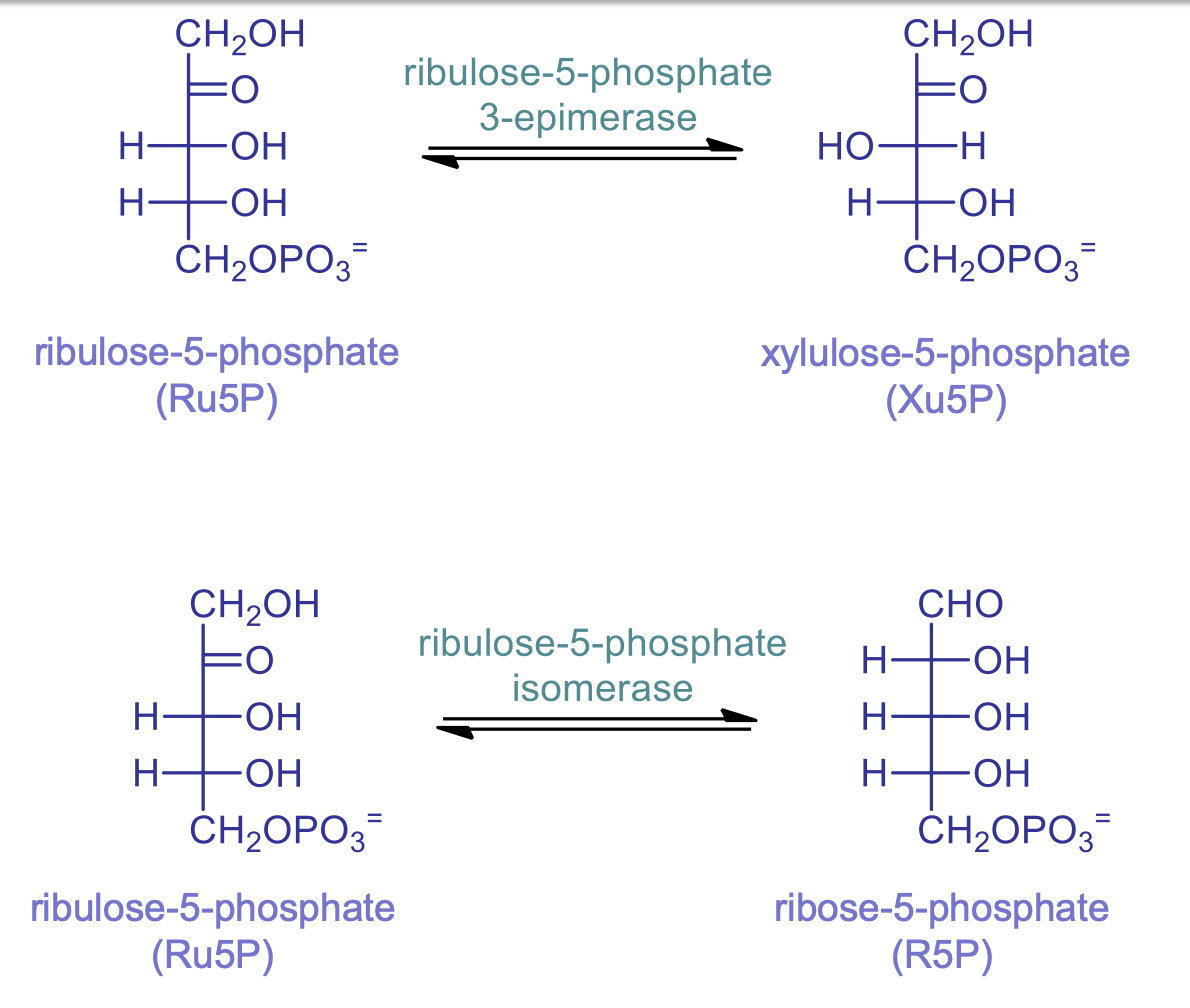
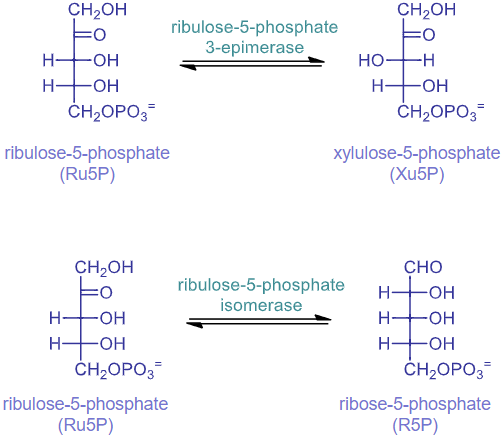
rxn 5: Ru5P epimerase
substrate
enzyme
product
overall, what occurs?
catalyzed by ribulose-5-phosphate 3-epimerase
switching stereoisomers
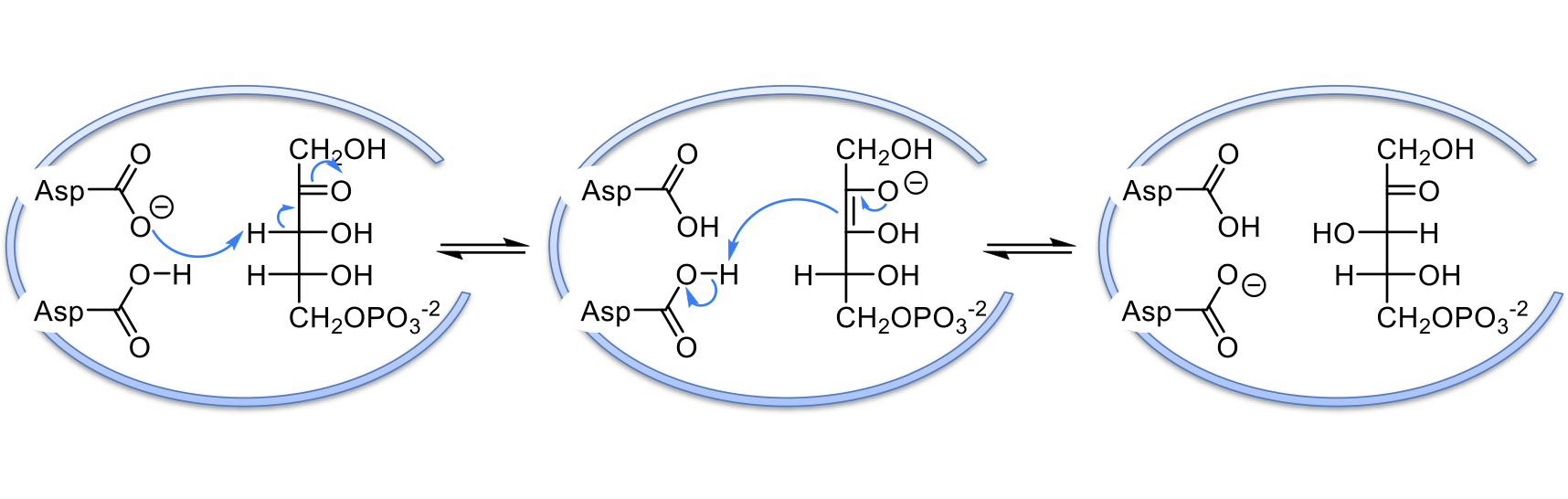
mechanism of epimerase
a negatively charged Asp residue in the active site acts as base and takes the acidic proton on carbon 3 of ribulose-5-phosphate. This proton is easily taken because of the carbonyl resonance
an enediolate intermediate is formed which takes a proton off of the other Asp residue. this proton is on the opposite face of carbon 3 than before
xylulose-5-phosphate is made (stereoisomer was changed)
mechanism of isomerase
A Glu residue in the active site takes a proton from Ribulose-5-phosphate’s carbon 1. This shifts electrons to form a carbon-carbon double bond between carbons 1 and 2. The carbonyl on carbon 2 steals a proton from an Asp residue
an enediol intermediate is formed which a double bond between carbons 1 and 2. the double bond is used to take back its H from Glu, but this time it bonds to carbon 2. Asp takes back its proton from carbon 1 so it can form a carbonyl.
ribose-5-phosphate is formed (the carbonyl switched positions)
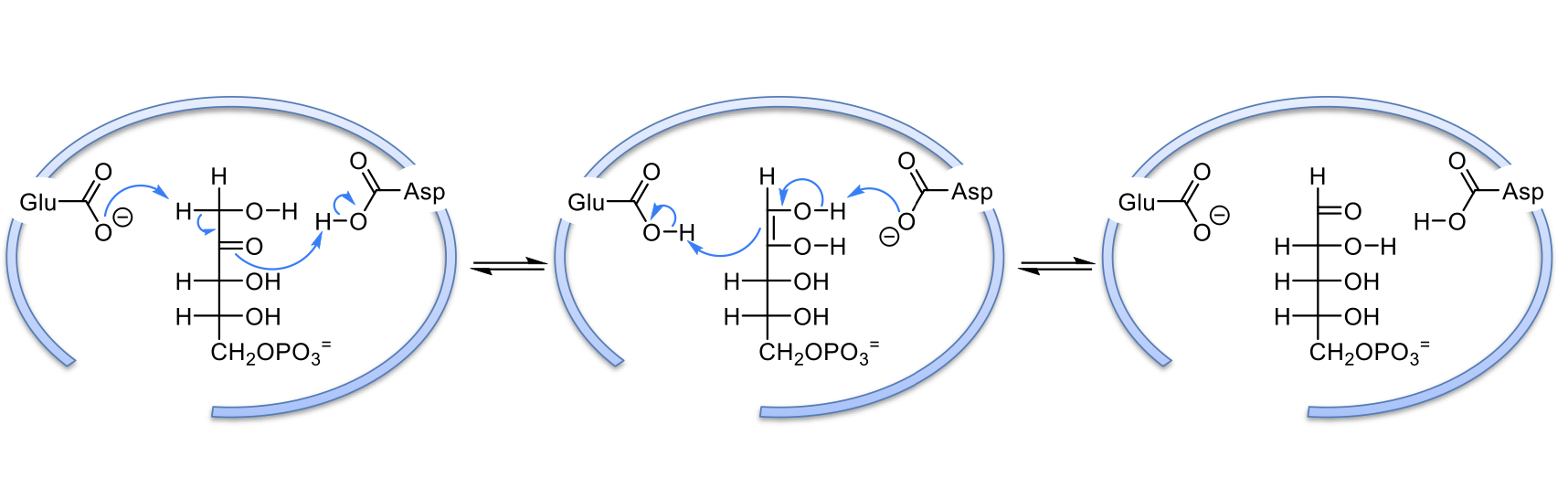
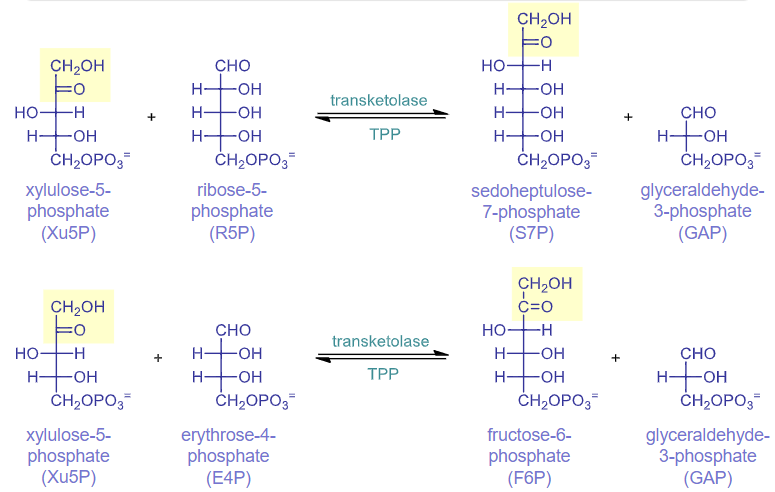
rxns 6 & 8: transketolase
cofactor required and why
catalyzed by transketolase
needs TPP because it stabilizes the carbanion of the 2 carbon unit that gets transferred from once substrate to the other
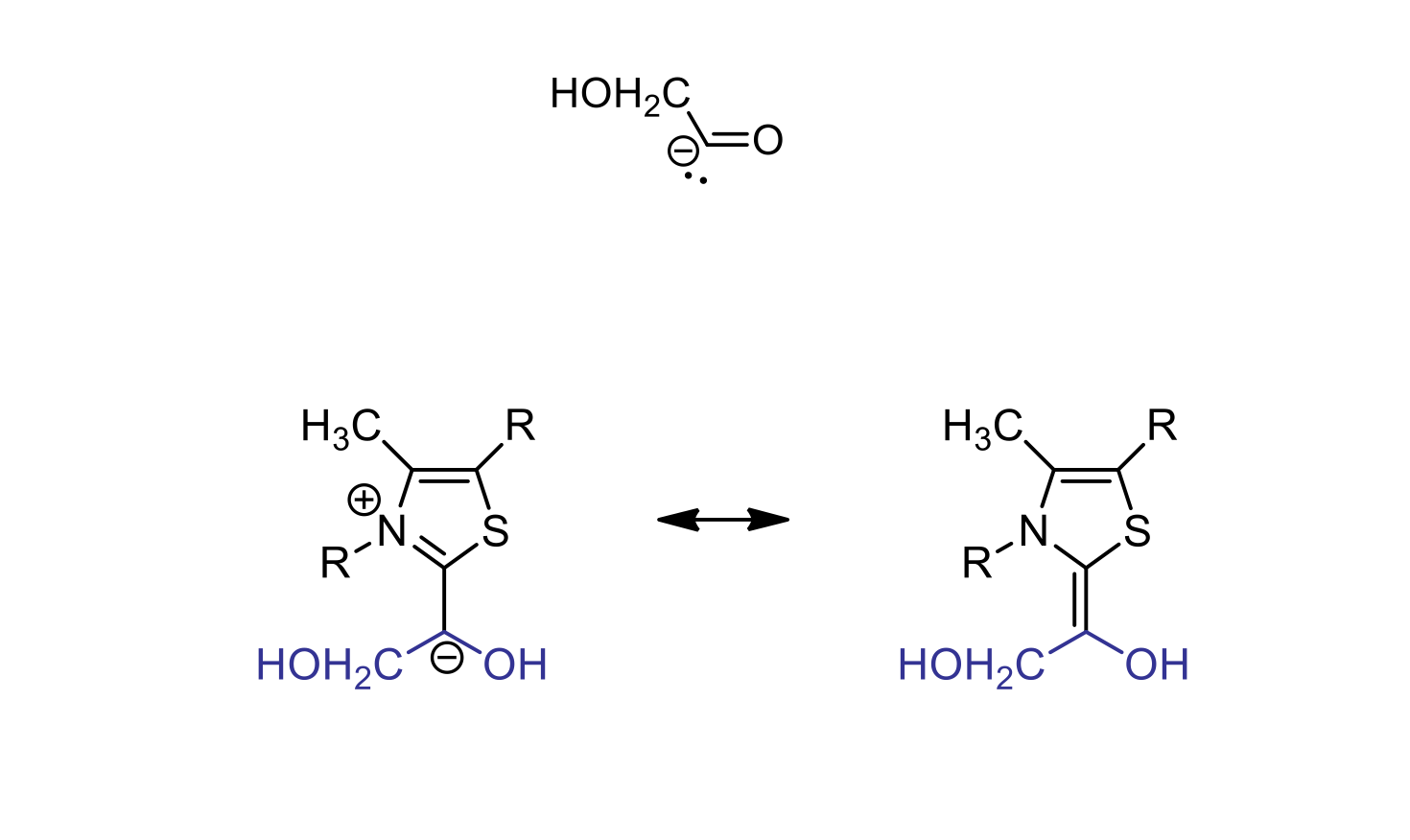
anion stabilization by TPP in the transketolase mechanism
TPP helps to stabilize the negatively charged intermediate formed during the reaction catalyzed by transketolase
essential for proper progression of transketolase reaction
TPP has a positive charge so it can take the electrons from the unstable 2 carbon unit
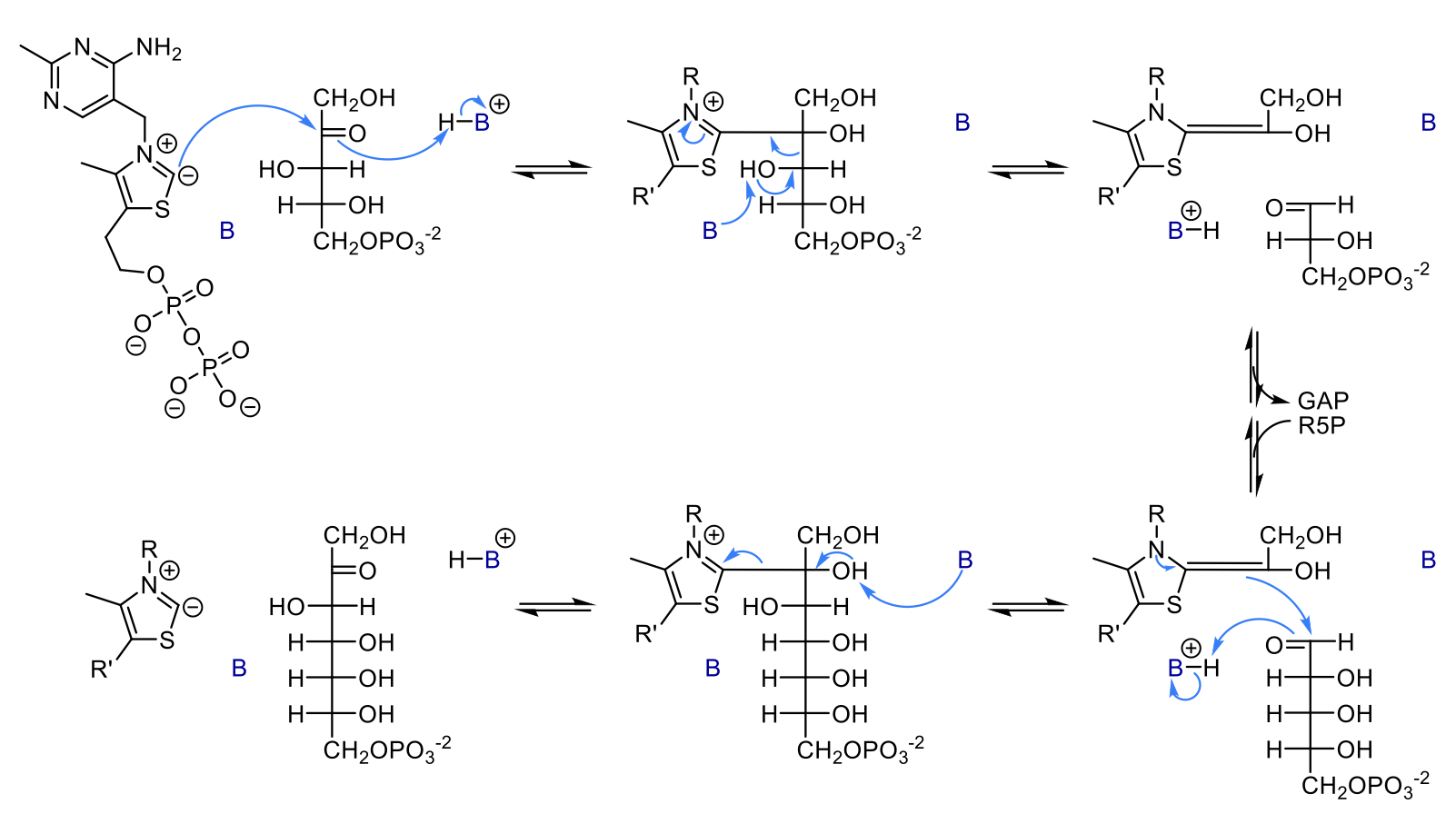
mechanism of transketolase
The negative charge on the active site TPP acts a a nucleophile and attacks the slightly positive C of the substrate’s carbonyl. the carbon of the carbonyl will then get a H from a nearby base.
the TPP is bound to the sugar and pulls electrons from it to stabilize it. The OH on the alpha carbon gives up its proton to a base and forms a carbonyl. this causes the bond between carbons 2 and 3 to break. These electrons are added to the sugar-TPP bond, producing the 2 carbon unit which is doubly-bound to TPP and GAP.
GAP leaves the active site, R5P or E4P (whatever is next substrate) comes into active site
electrons from the double bond between the 2-carbon unit and TPP are used to form a bond with the new substrate by attacking the carbonyl. the oxygen of the carbonyl (on carbon 3) picks up a H to form OH
a base takes a H from the OH group on carbon 2 so it can form a carbonyl. This causes the bond between TPP and the carbon to break.
product is formed and TPP is back to normal with one positive charge and one negative charge, ready for next reaction
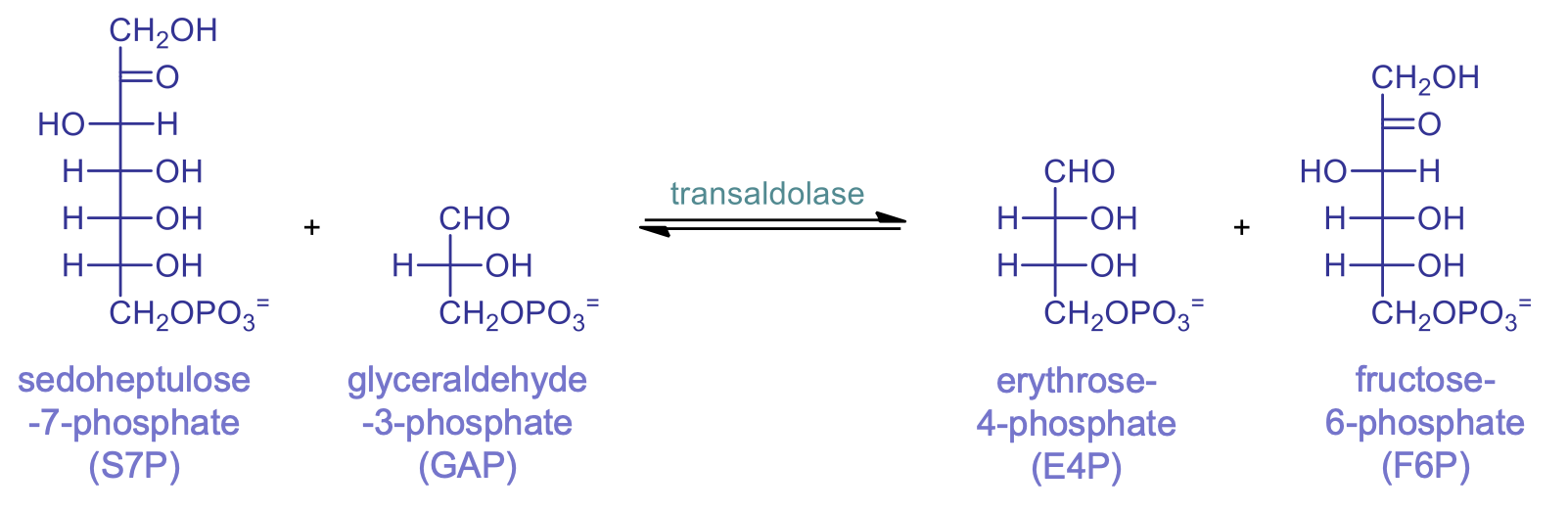
rxn 7: transaldolase
products and where than can go
overall, what happens
E4P and F6P are made. E4P stays in the pentose phosphate pathway and F6P enters glycolysis
bond between alpha and beta carbons breaks, 3 carbon unit is transferred to a different aldehyde
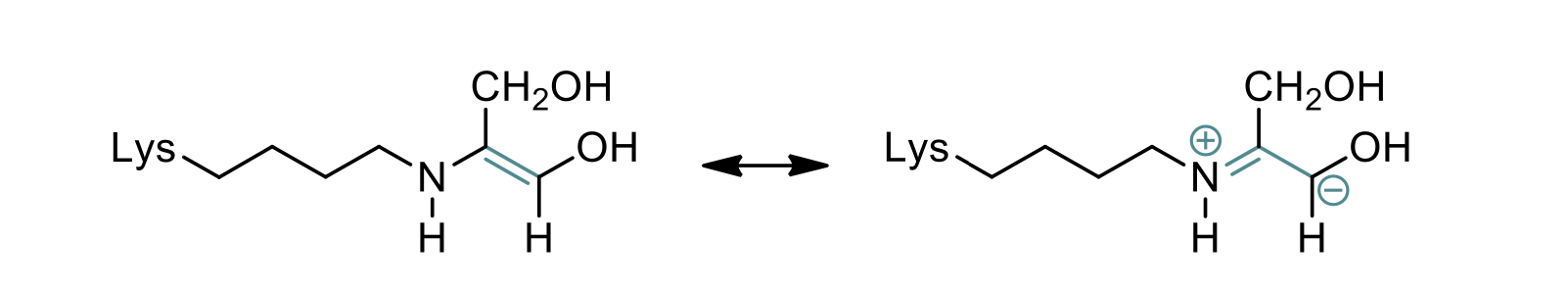
anion stabilization by a schiff base in the transaldolase mechanism
what is a Schiff base
what does it do?
schiff base is a chemical structure formed by a nitrogen atom from a lysine residue in the enzyme and a carbonyl group from the substrate
stabilizes the negative charge on the intermediate
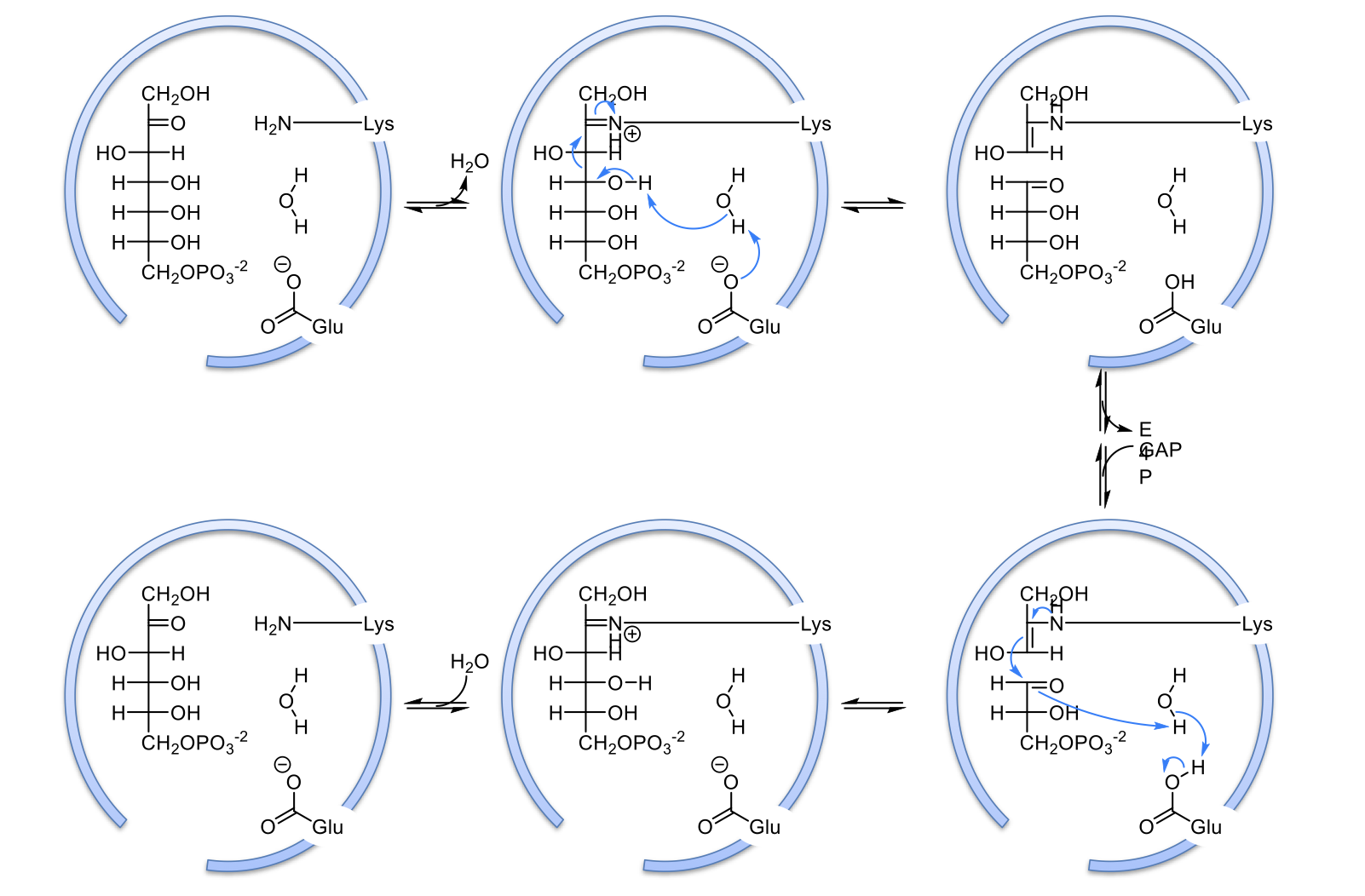
mechanism of transaldolase
lot’s of steps that basically ends with carbonyl on carbon 2 being replaced with N from lysine in active site
Glu residue activates H2O as a nucleophile by taking one of its Hs. this forms OH which attacks the OH group on carbon 4 to steal its H. This causes a carbonyl to form at carbon 4/beta carbon and causes a break in the bond between alpha and beta carbons
E4P is formed and released so the GAP has room to come into the active site. Lysine is attacked to the 3 carbon intermediate
the double bond on the 3 carbon intermediate attacks GAP’s carbonyl to form a bond. The nitrogen from Lysine from a double bond with the 3 carbon unit
water come in to form a hydrolysis reaction in which the N double bond is replaced with an oxygen to reform the carbonyl and return lysine to normal
overall energy yields of pentose phosphate pathway
If pathway stops after R5P formation (only need R5P so don’t continue pathway):
G6P + 2 NADP+ +H2O → R5P + CO2 +2 NADPH + 2 H+
If pathway is completed (don’t need R5P):
6 G6P+ 12 NADP+ → 5 G6P + 6CO2 + 12 NADPH + 12 H+ + Pi
because of the 12 NADPH, the energy pay off is much more
since pentose phosphate pathway is not a major E source, when is it used?
only when we need NADPH or ribose-5-phosphate
regulation of pentose phosphate pathway
when cell needs more NADPH or ribose-5-phosphate, G6PDH gets more active
it is allosterically activated by NADP+
NADPH inhibits G6PDH by product inhibition.