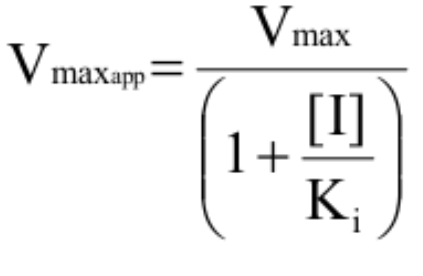Biochem Lec 11-Enzymes III: Inhibition
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What are the 3 types of reversible inhibitors?
1) Competitive (most common)
2) Uncompetitive
3) Noncompetitive
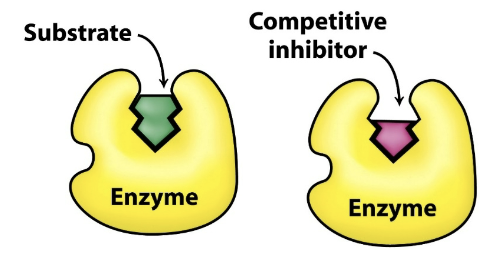
Where does a competitive inhibitor bind?
Binds reversibly to the active site of the enzyme → forms a nonproductive enzyme inhibitor complex (EI)
The substrate and inhibitor compete for binding at the active site
What is Ki?
The equilibrium dissociation constant that defines the affinity of I for the enzyme. The higher the affinity, the more potent the inhibitor.
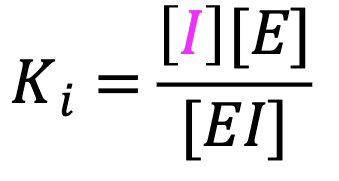
What is the relationship between Ki and the affinity of the inhibitor for the enzyme?
Low Ki → high affinity
High Ki → low affinity
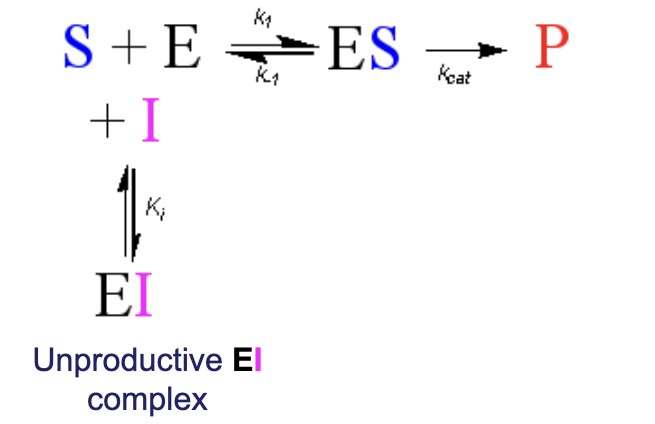
How does the unproductive EI complex fit into the enzyme/substrate to product reaction?
It adds to the beginning with E and S and is in equilibrium. It competes with the formation of ES.
What variables do competitive inhibitors impact?
Increase [S] needed to reach ½ Vmax → increase KM (KM<KMapp)
DO NOT affect Vmax
What is the easiest way to identify competitive inhibition and calculate Ki?
Lineweaver-Burk plots because they make it easy to visualize how competitive inhibitors affect KM (change X-int) but do not affect Vmax. Secondary plot can be made of the slopes vs. [I]→ Ki is negative reciprocal of this X-int
What is KMapp and how is it calculated (competitive)?
M-M rate constant with inhibitor
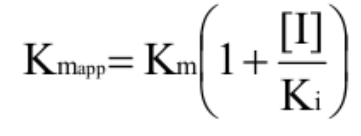
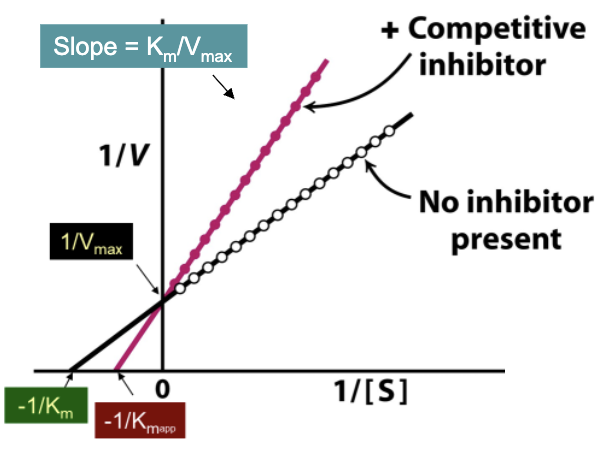
How do the Lineweaver-Burk plots of enzymes with and without competitive inhibitors differ? How are they the same?
With competitive inhibitor:
Different slope
Different X-int → larger KM (KMapp)
Y-int stays the SAME → no effect on Vmax
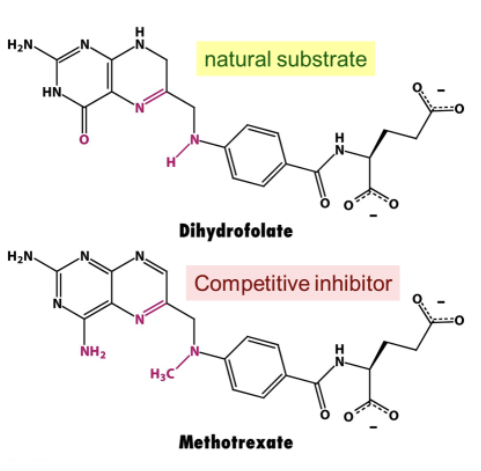
How do drugs that are competitive inhibitors compare to substrates?
They are structurally similar:
Bind with similar chemical complementarity as the substrate to the enzyme active site
Provide an example of a drug that is a competitive inhibitor. How does its structure compare to the natural substrate?
Methotrexate (anti-cancer) → target dihydrofolate reductase, an enzyme involved in nucleotide biosynthesis in tumor cells.
Very similar structure → only differs in 2 places
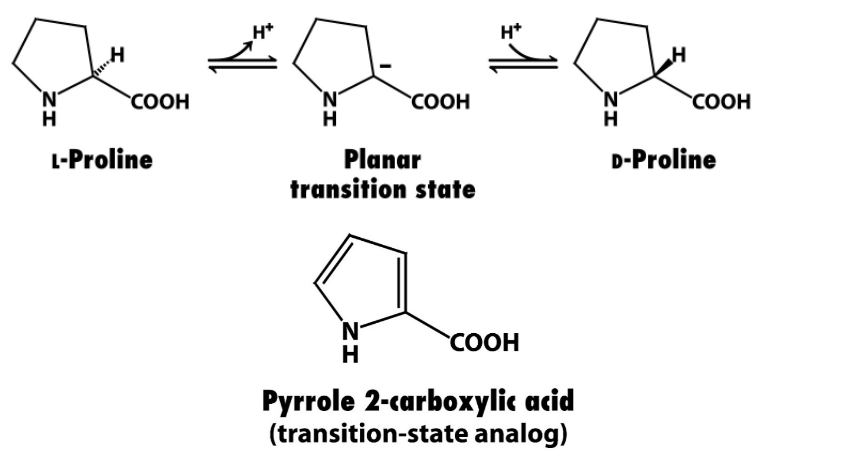
What are competitive inhibitors often modeled after and why?
The transition state (X‡) because enzymes often have a higher affinity for the transition state.
Why do enzymes often have a higher affinity for the transition state?
Enzymes often have higher affinity for the transition state than for the substrate because stabilizing this high-energy state lowers the activation energy and speeds up the reaction. If they bound the substrate more tightly, it would actually hinder catalysis by trapping it in the ground state.
Provide an example of an enzyme that binds more strongly to the transition state and explain.
Proline racemase:
Ki for pyrrole 2-C (transition state analog) is 160x lower than the KM for proline → the transition state analog binds much more strongly to the enzyme
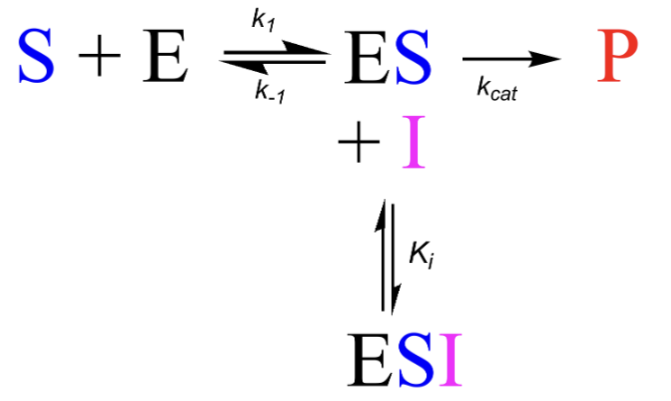
Where do uncompetitive inhibitors bind?
Uncompetitive inhibitors bind selectively to the ES complex, often adjacent to the active site → A ternary unproductive complex of ESI is formed
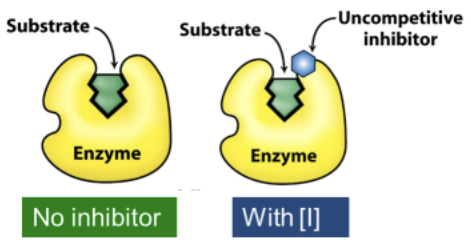
How to uncompetitive inhibitors affect the reaction of substrate/enzyme to product?
They are added to the ES and form an equilibrium with ES and ESI
What variables to uncompetitive inhibitors affect?
KM AND Vmax → lower
KM → binding affinity appears greater for substrate because the substrate stays trapped in ESI complex
Vmax → prevents ES from completing the reaction, so fewer active complexes make product
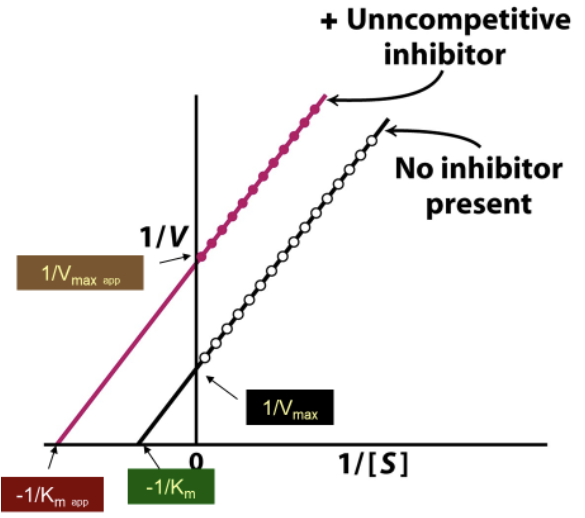
What do uncompetitive inhibitors NOT affect? How does this affect the Lineweaver-Burk plot?
The ratio of Km/Vmax stays the same → slope does not change
How does the Lineweaver-Burk plot with/without an uncompetitive inhibitor compare?
With uncompetitive inhibitor:
Larger Y-int → lower Vmax
Smaller/more negative X-int → lower KM (KMapp)
Slope is the same for both
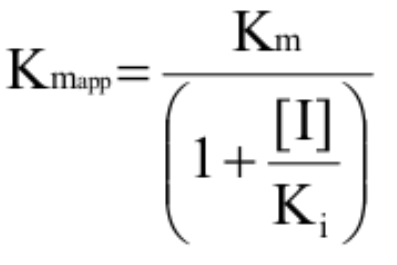
How is Kmapp calculated (uncompetitve)?
How is Vmaxapp for uncompetitive inhibitors calculated?
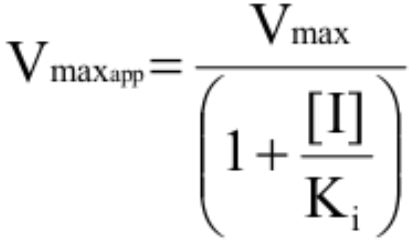
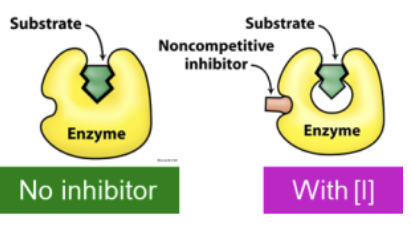
Where do noncompetitive inhibitors bind?
Noncompetitive inhibitors bind independently of the S to the E resulting in an unproductive complex (either EI or ESI)
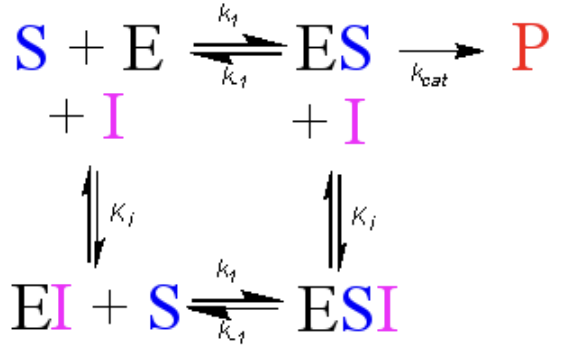
What variables to noncompetitive inhibitors affect?
Lowers Vmax
No effect on KM
How do noncompetitive inhibitors affect the reaction of enzyme/substrate to product?
Added to both S and E and ES. Forms equilibrium with ES to form ESI → in equilibrium with EI+S → in equilibrium with S+E
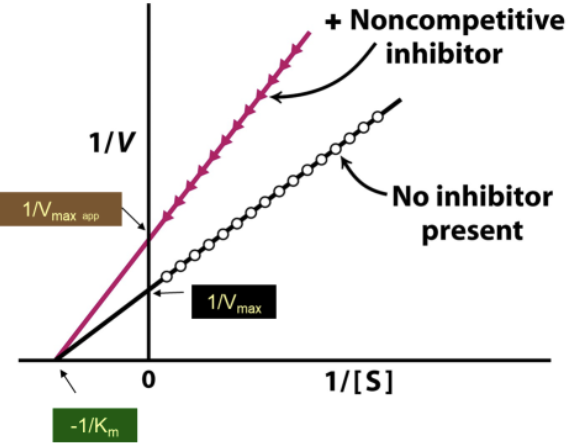
How do the Lineweaver-Burk plots of with/without a noncompetitive inhibitor compare?
With noncompetitive inhibitor:
Larger Y-int → lower Vmax
Same X-int → no effect on KM
How is Vmaxapp calculated for noncompetitive inhibitiors?