Organic Chemistry Nomenclature
1/58
Earn XP
Description and Tags
Rules for naming organic compounds
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
prefix
what part of the name tells you about how many carbon atoms are present?
meth-
1 carbon
eth-
2 carbons
prop-
3 carbons
but-
4 carbons
pent-
5 carbons
hex-
6 carbons
hept-
7 carbons
oct-
8 carbons
non-
9 carbons
dec-
10 carbons
undec-
11 carbons
dodec-
12 carbons
tridec-
13 carbons
tetradec-
14 carbons
pentadec-
15 carbons
hexadec-
16 carbons
heptadec-
17 carbons
octadec-
18 carbons
nonadec-
19 carbons
eicos-
20 carbons
Infix
what part of the name tells you about the nature of the carbon-carbon bonds?
-an-
all single bonds
-en-
one or more double bonds
-yn-
one or more triple bonds
suffix
what part of the name tells you the class of compound?
-e
hydrocarbon
-ol
alcohol
-al
aldehyde
-amine
amine
-one
ketone
-oic acid
carboxylic acid
parent name
the longest carbon chain
substituent
a group bonded to the parent chain
alkyl group
a substituent derived by removal of a hydrogen from an alkane; given the symbol R- (Radical) and suffix -yl
find the longest continuous carbon chain
what is the first step in nomenclature?
base alkane name
what does the number of carbons in the longest chain determine?
most substituents
if there are two possible chains with the same number of carbons, use the chain with the ____ ____________.
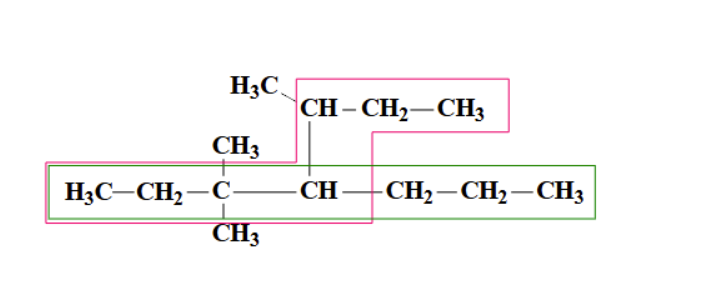
pink
what chain would you use? (pink or green)
the end closest to the first attached group
where do you start counting the parent chain?
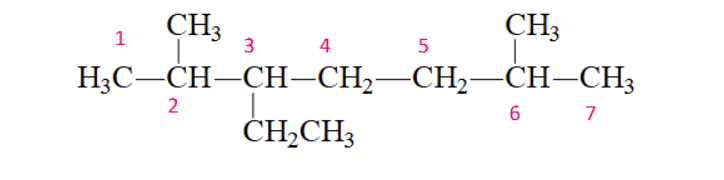
at the end nearest to the second branch point
if you are counting your carbons and both ends have a substituent at the same distance, where do you start?
alkyl group
each substituent is given a name and a number. how should you name the substituent groups attached to the longest chain? keep in mind, they would be a group with an H removed.
1-methylethyl (isopropyl)
what is the name of this substituent group?
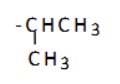
2-methylpropyl (isobutyl)
what is the name of this substituent group?
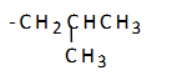
1-methylpropyl (sec-butyl)
what is the name of this substituent group?
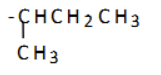
1,1-dimethylethyl (tert-butyl)
what is the name of this substituent group?
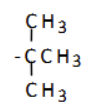
the end that gives the lower number to the substituent encountered first
if there are two or more identical substituents, which end should you number from?
use a prefix to indicate how many times it appears
if there are two or more identical substituents, how should you indicate that?
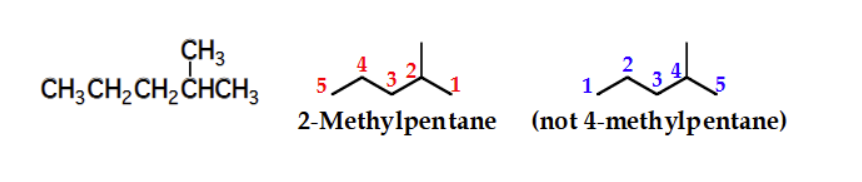
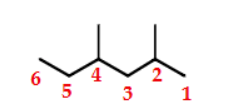
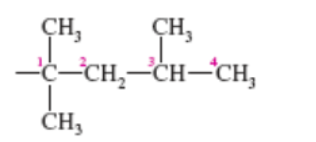
2,4-dimethylhexane
name this molecule
list them in alphabetical order
what do you do if there are two or more different substituents?
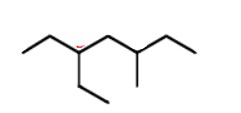
3-ethyl-5-methylheptane
name this molecule
number, alkyl, parentheses
what if there is a complex substituent (a branch has a branch)?
_____ the carbons from the point of attachment
name the branch off the branch as an ____ group
_______ are used around the complex branch name
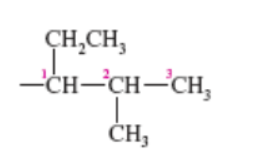
(1-ethyl-2-methylpropyl)
name this complex substituent
(1,1,3-trimethylbutyl)
name this complex substituent
cycloalkanes
what is it called when there is a ring of carbon atoms?
no need; lower alphabetical; lowest, alphabetical order
naming cycloalkanes:
if only one substituent, __ ____ to give it a number
if two substituents, number from the substituent of _____ _________ order
if three or more substituents, number to give them the ______ set of numbers and then list substituents in ________ ____
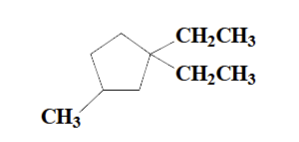
1,1-diethyl-3-methylcyclopentane
name this molecule
T
(T/F) If an alkyl chain contains more carbons than an attached cycloalkane, then name it as an alkane with a cycloalkyl substituent.
2-cyclopentylheptane
name this molecule