Specific heat capacity
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
What is specific heat capacity?
the amount of energy (heat) required to increase the temperature of 1kg of a substance by 1K (kelvin).
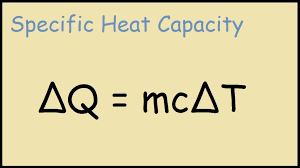
What is the formula for the amount of heat energy (q) gained or lost by a substance/
mass of substance (m) x specific heat capacity (C) x change in temperature (triangleT)
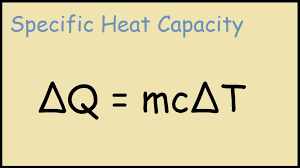
What is the formula for heat capacity?
Mass (m) X Specific heat capacity (c).
What is the latent heat of fusion/enthalpy of fusion?
the amount of energy that must be supplied to a solid substance (typically in the form of heat) in order to trigger a change in its physical state and convert it into a liquid (when the pressure of the environment is kept constant)
What is the specific latent fusion of a substance?
the heat energy needed to change a unit mass of a substance from solid to liquid without temperature change.