Chapter 25: Aromatic Chemistry
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Write a detailed summary to explain why the delocalised model of benzene is more accurate than kekule’s model of benzene
based on kekule’s model of benzene, the theoretical enthalpy change of hydrogenation of benzene was expected to by -360 kJmol-1. However, the experimental enthalpy change was -208 kJmol-1. Therefore, benzene was found to be 152 kJmol-1 more energetically stable than expected. This is because the delocalised π electrons in the delocalised model of benzene increases the stability of benzene and more heat energy is required to disrupt this stable π system
the delocalised π system in the delocalised model of benzene means that benzene has a lower electron density compared to Kekule’s model. This explains why benzene is resistant to electrophilic addition reactions and doesn’t decolourise in bromine water as benzene is unable to induce a dipole in bromine or other electrophiles
if 6 π electrons in benzene were localised as shown in Kekule’s model, then benzene would contain two different bond lengths (a s ingle and double carbon-carbon bond length). However, x-ray crystallography highlights that actual benzene contains carbon-carbon bonds all of the same length. This suggests the 6 π electrons in benzene must be delocalised between all 6 carbons, which is illustrated in the delocalised model of benzene
Alkenes and benzene both react with bromine but alkenes are much more reactive. Explain the relative resistance to bromination of benzene compared with alkenes.
In benzene, π electrons are delocalised
In alkenes, π electrons are localised
Benzene has a lower electron density OR alkenes have a higher electron density
Benzene induces a weaker dipole in bromine/attracts bromine less
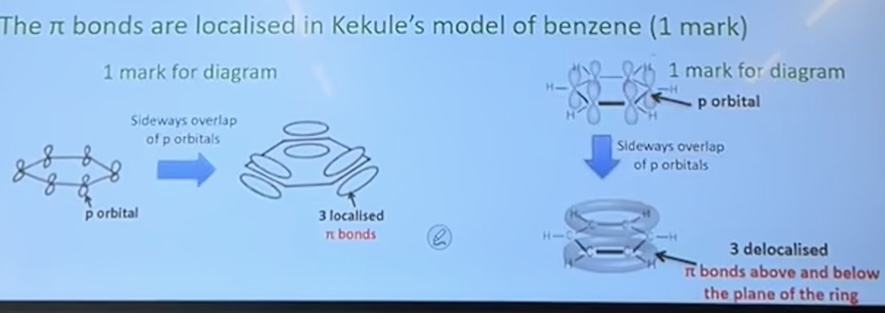
Describe, with aid of suitable diagrams showing orbital overlaps, the difference in bonding between Kekule’s model of benzene and the delocalised model of benzene
In both Kekule’s model of benzene and the delocalised model of benzene, the p-orbitals overlaop to form π bonds
the π bonds are delocalised in the delocalised model of benzene
the π bonds are localised in Kekule’s model of benzene
Name for side chains attached to R group:
NO2
NH2
CN
OH
nitro
amino
cyano
hydroxy
Name these compounds
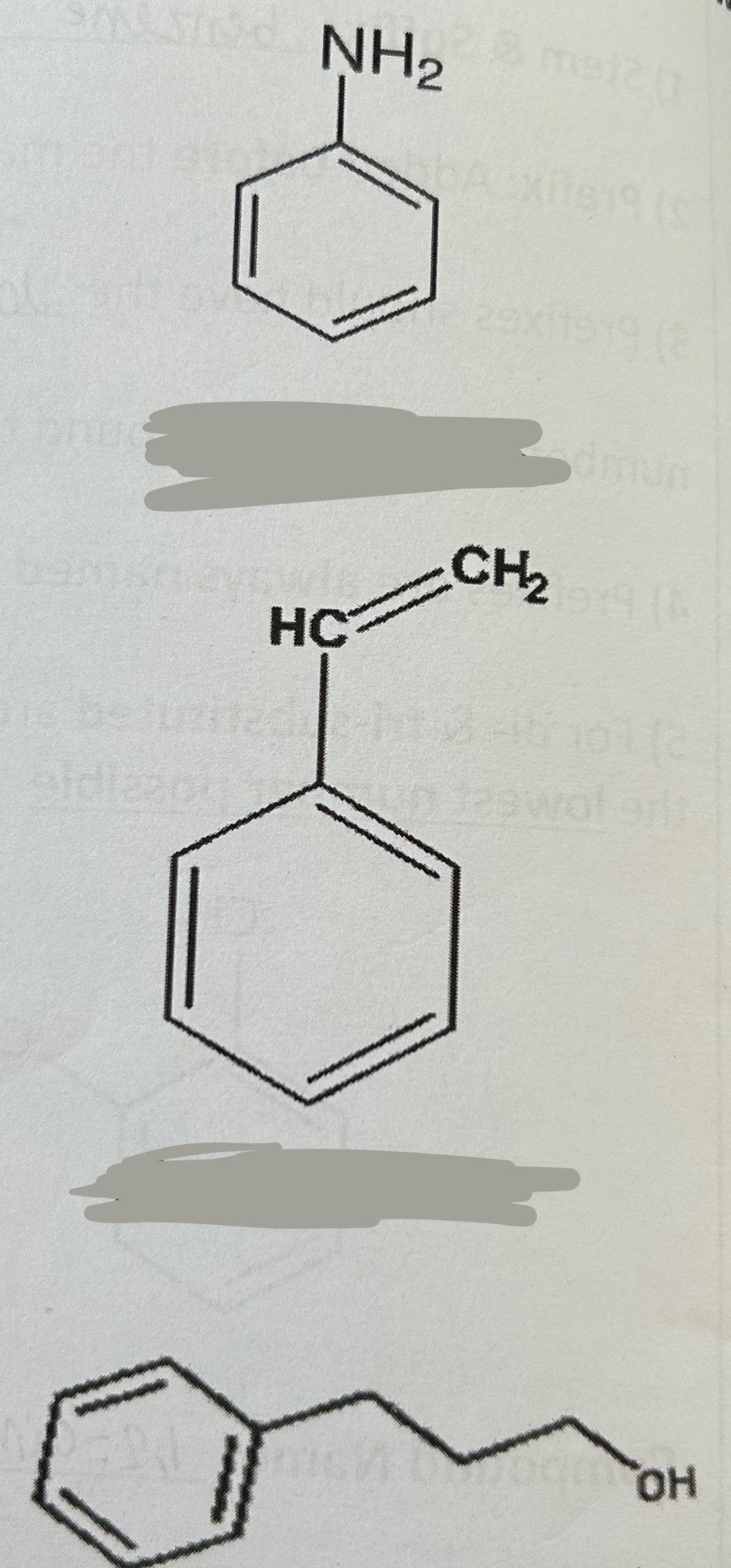
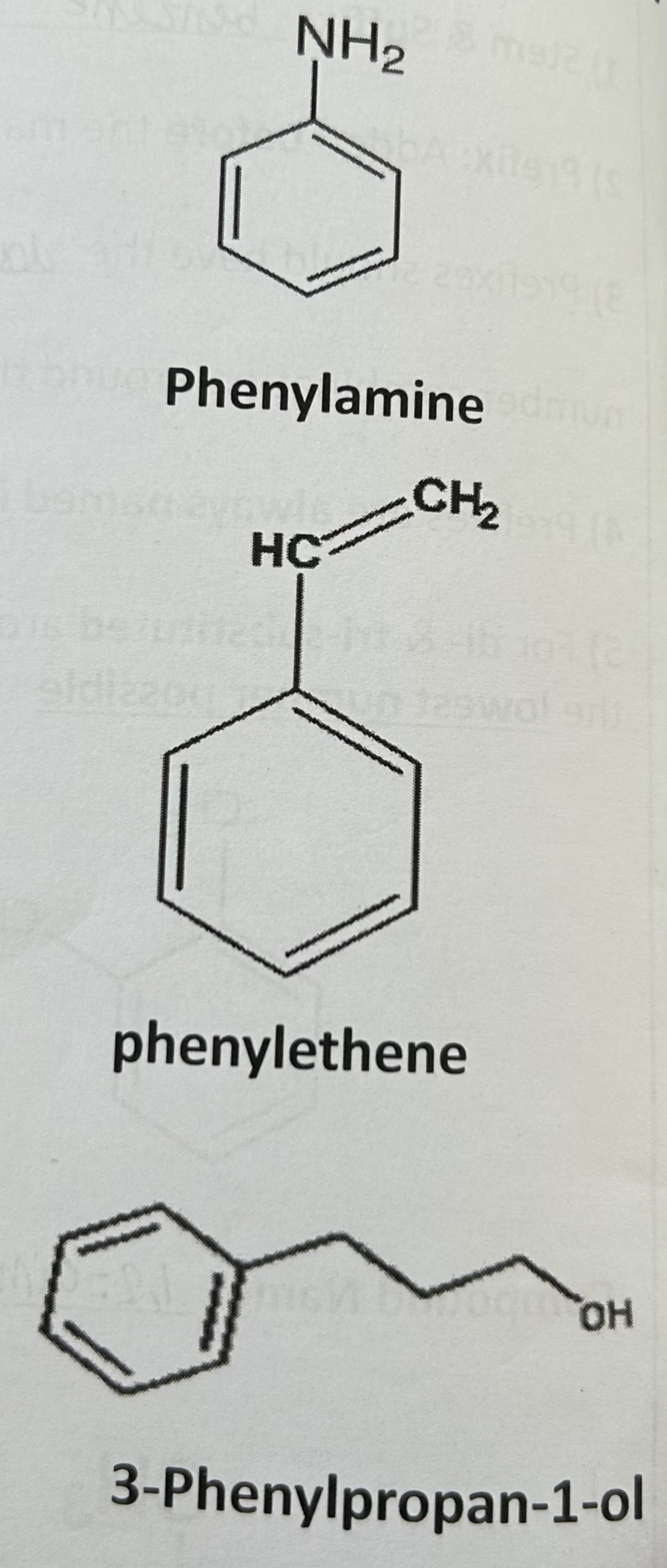
Name these compounds
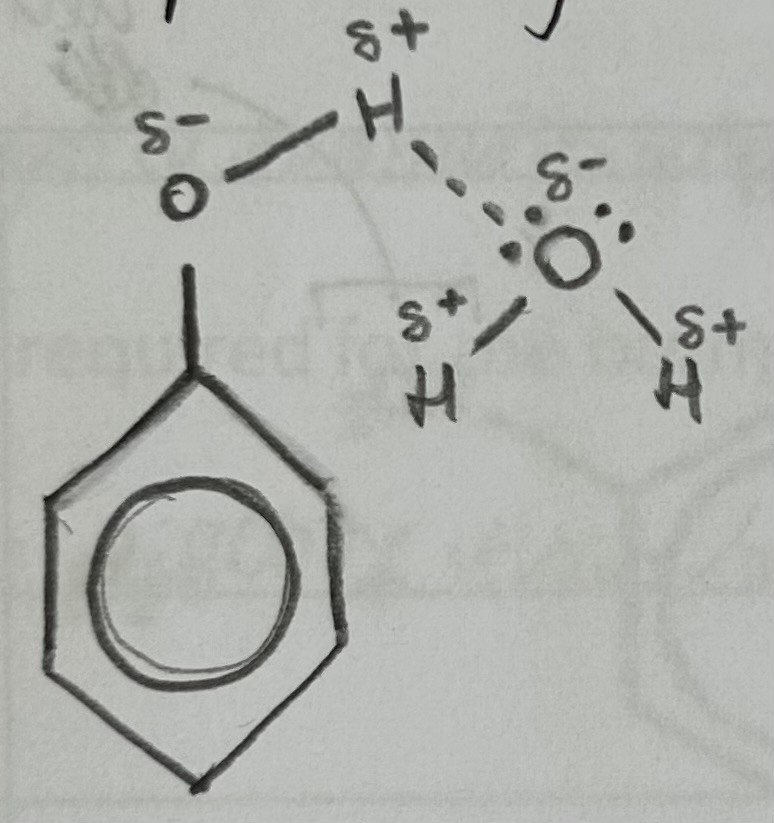
State and explain Phenol’s solubility in water
Sparingly soluble
hydroxyl group in phenol can form hydrogen bonds with water molecules, but aromatic ring is non-polar and therefore cannot form hydrogen bonds with water, making it partially soluble
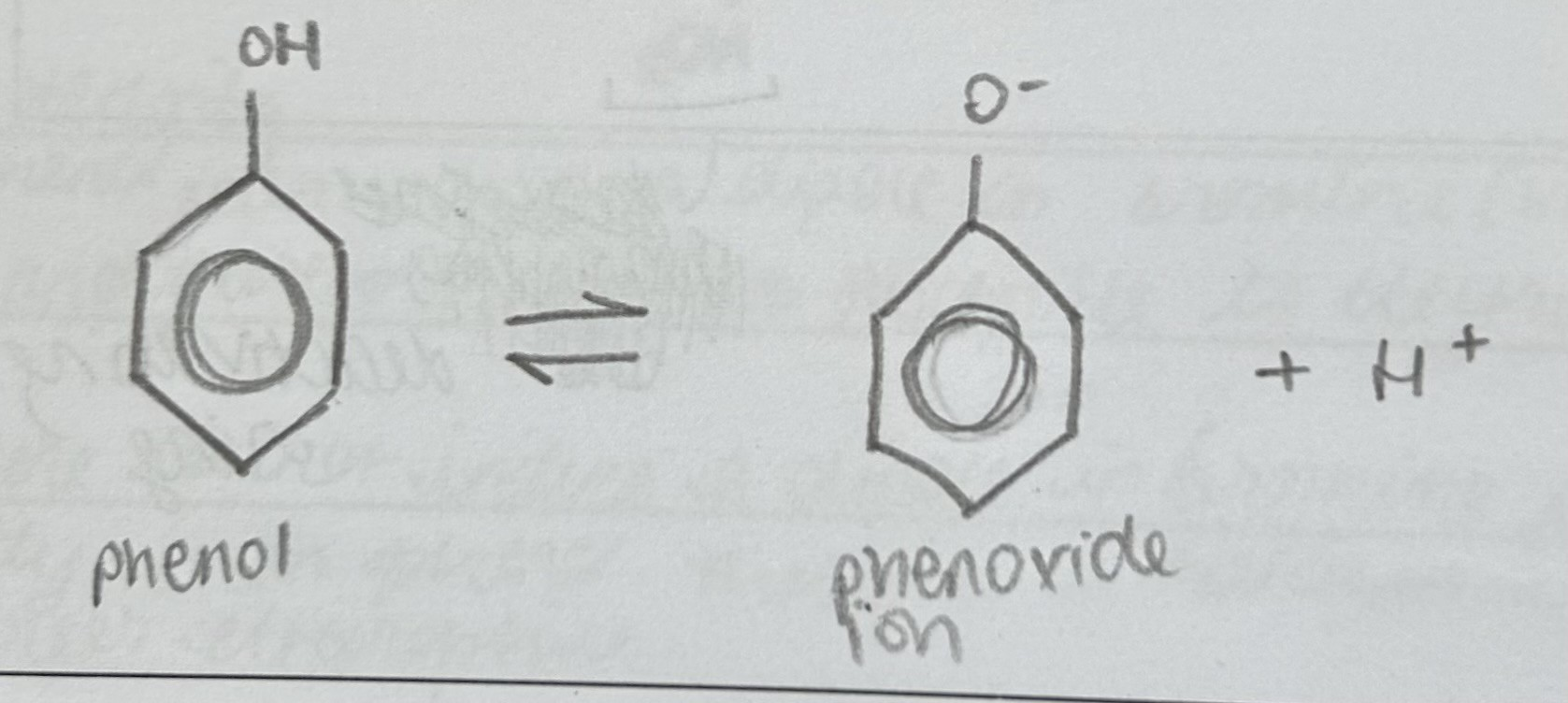
State and explain the property of Phenol when Universal Indicator is added
Turns orange
phenol is a weak acid with a pH of 5-6 and therefore turns universal indicator a orange colour. It partially dissociates in aqueous solution, therefore there is a higher concentration of phenol compared to the phenoxide ion
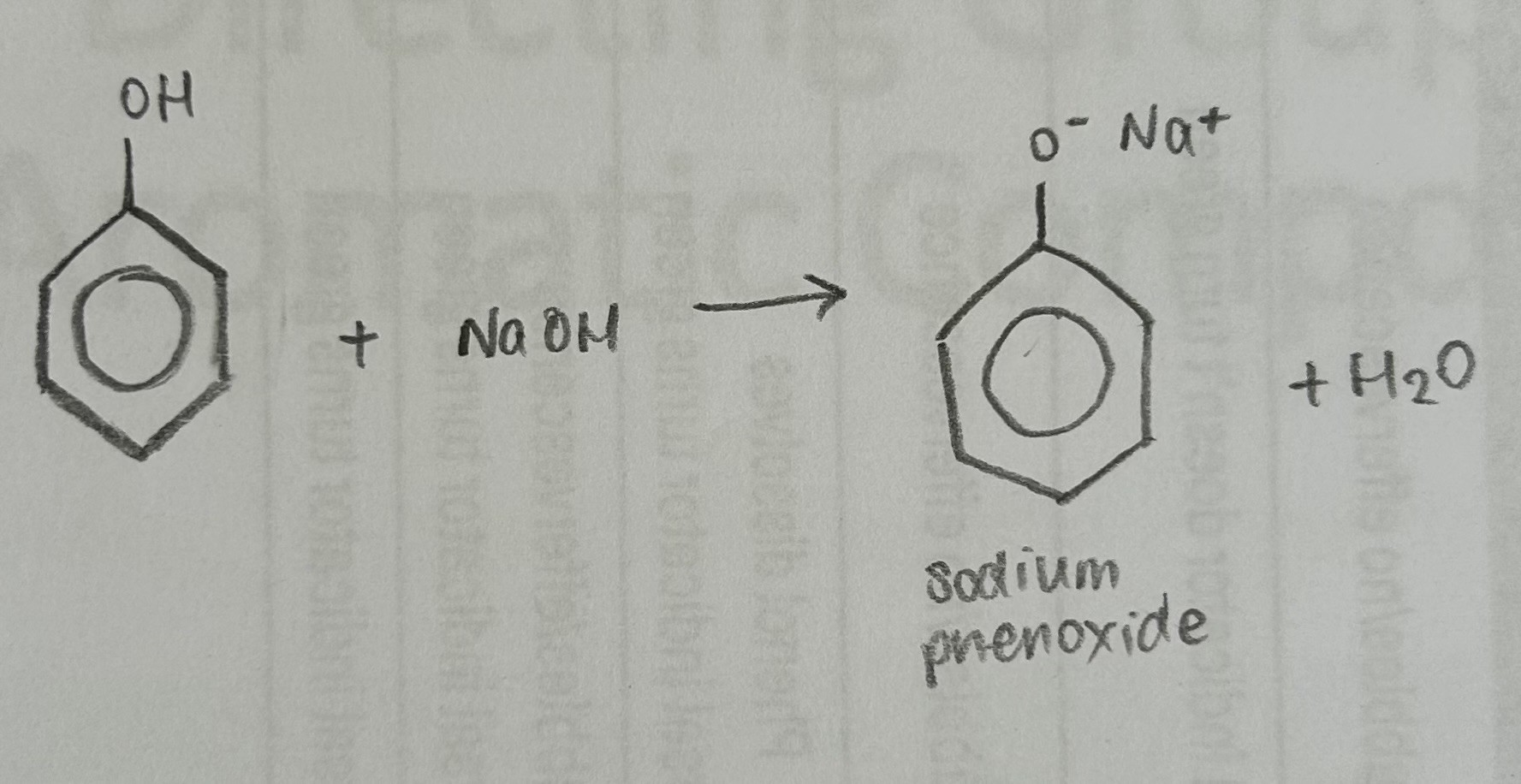
State and explain the property of Phenol when NaOH solution is added
Phenol completely dissolves
Phenols can react with strong bases such as sodium hydroxide to form a soluble salt in water. As a result, phenol dissolving in sodium hydroxide is observed, and universal indicator can be used to evidence neutralisation reaction took place (indicator turns green)
(phenol is a weak acid so cannot react with weak bases such as sodium carbonate or calcium carbonate)
State and explain the property of Phenol when bromine is added
foes from orange to colourless and a white precipitate is formed
An orange to colourless solution is observed which shows that bromine is reacted with. A white precipitate is observed which shows that 2,4,6-tribromophenol is produced.