c: unit 2, topic 2 & 3 - aqueous solutions and molarity + rates of chemical reactions
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
what is the structure of water
UB 2HO ED H
unsymmetrical
bent polar molecule
2 polar O-H bonds
hydrogens: electron deficient
oxygen: electron rich
electronegativities: electron pairs are shared unequally due to large difference in electronegativities
dipole: can have dipole-dipole interactions with itself or other polar compounds
hydrogen: main bonds are hydrogen
are water particles more or less dense when solid compared to liquid? explain why
less dense
crystal: ice forms a tetrahedral crystalline structure that is less dense than the liquid, so it floats
9%: expands approximately 9% by volume
are all water molecules hydrogen bonded equally? explain why
no
the below bullet points create surface tension
AAS
attractions: in liquid water, each molecule has attractions in all directions and hydrogen bonds, except for the water molecules at the surface
air: surface molecules are exposed to the air above and are only attracted to other water molecules on the sides and below
skin: this imbalance causes surface molecules to be pulled inward and closer together, minimising the surface area and creating a skin-like effect at the surface
this is responsible for the meniscus formed in a glass of water
surface tension definition
the ability of the surface of a liquid to resist an external force (because of the strong intermolecular interactions)
why is water’s boiling temperature so high
because of the intermolecular hydrogen bonds holding water molecules together
definition of solute, solvent & solution
solute: the substance that is dissolved in a solvent
solvent: the substance that dissolves a solute
solution: formed when a solute dissolves in a solvent
the solvent and solute particles are evenly spread throughout the solution and can’t be distinguished from one another
why is water the best solvent
easily dissolves polar covalent compounds
ionic compounds
why do most ionic compounds easily dissolve in water?
dissociation process
water molecules orientate themselves so that they interact with the oppositely charged ion of the solute
water molecules ‘pull’ the ions, separating them form the solute, forming ion-dipole interactions
definition of an ion-dipole interaction (section: why water is the best solvent)
electrostatic attraction between an ion and a molecule that has a dipole
what are the 3 steps that need to occur for any substances to dissolve in a solvent
solvent-solvent forces broken
solute-solute forces broken
solute-solvent forces form
what is concentration (in chem)
the amount of solute in a given volume of solvent, typically expressed in mol L^-1 or M or square brackets []
concentrated solution: large ratio of solute compared to solvent
dilute solution: large ratio of solvent compared to solute
define dissociate (in terms of chem solutions)
the process by which substances separate into smaller particles in solutions
difference between acid strength and acid concentration
acid strength: the degree of dissociation in water
acid concentration: the amount of solute in a given volume
saturated, unsaturated and supersaturated (solutions)
saturated: can’t dissolve any more solute as it contains the maximum amount of solute
unsaturated: can dissolve more solute as it contains less than the maximum amount of solute
supersaturated: containing more than the maximum amount of solute that can be dissolved
achieved through temperature increase, and then very slow cooling
formulas for calculating concentration
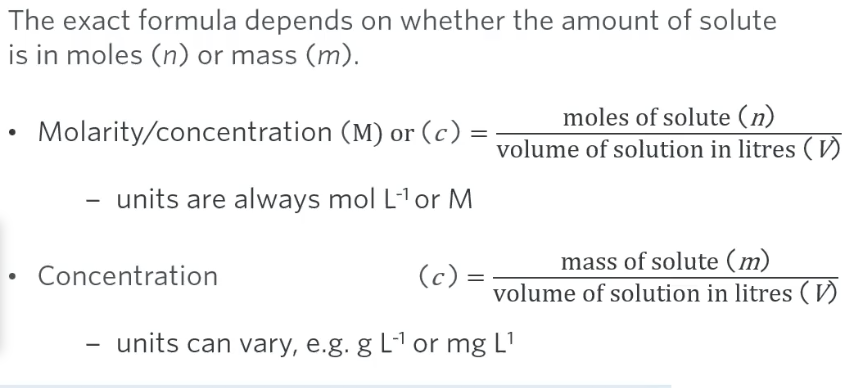
definition of anion & cation
anion: a negatively charged ion
cation: a positively charged ion
what is pH
A1 HH >7 LHL
acidity: used for determination of the alkalinity or acidity of a substance1-<7: acidichigher concentration of hydrogen ionshydrogen: soluble substances that dissociate in water to form hydrogen/hydronium ions
>7-14: basic (alkalis)lower concentration of hydrogen ionshydroxide: soluble bases that dissociate in water to form hydroxide ions
log: is a log scale. each value is a whole log bigger than the last
indicator definition (in relation to pH)
a substance that changes colour to indicate a pH range
what is the arrhenius model
describes the acid-base theory
what determines the strength or weakness of an acid/base
the degree of dissociation to form H+/OH- ions respectivelydifferent to concentration, which refers to the amount of solute in a given volume of a solution
is water an acid or a base?
BS
both: it can act as both a weak acid + base (amphiprotic)
self: because it can self-ionise
what is a neutralisation reaction
a reaction between an acid and alkali/base that forms a salt and awter (and carbon dioxide when a metal carbonate is a reactant)
what are the 4 types of neutralisation reactions
acid + metal hydroxide - salt + wateracid + metal carbonate - salt + water +CO2acid + metal hydrogen carbonate - salt + water +CO2acid + metal oxide - salt + water
what is the difference between an alkali and a base
alkali: typ eof base that is soluble in water
base: broader term for any substance that reacts with an acid
what 3 things must occur for a chemical reaction to occur
the reactants:
CAO
collide: physically collide with each other
activation: have sufficient activation energy
orientation: collide with a specific orientation
how can rates of reaction be increased
increasing the
FP
frequency of collisions
proportion of successful collisions
how does temperature affect the rate of reaction and why
temp increases, rate of reaction also increases
why?
KCC TP
kinetic: increases particles average kinetic energy,
causes:
collisions: more frequent collisions
threshold: the particles to exceed the activation energy threshold
proportion: increasing the proportion of successful collisions
how does surface area affect the rate of reaction and why
only has an impact if the reactant is a solid
sa increases, ror increases
increases the amount of exposed space that a reactant can collide with
increases frequency of collisions
how does pressure affect the rate of reaction and why
changing pressure only applies if the reactant is ag as
pressure increases, ror increases
by increasing pressure of gaseous molecules, there are more molecules in a smaller volume
increases the frequency of successful collisions and the ror
how does pressure affect the rate of reaction and why
only applies if a solution
concentration increases, ror increases
increases number of particles available for collision
increases total number of successful collisions
what is activation energy
the minimum amount of energy required for a reaction to proceed
required to break the chemical bonds in the reactants
not the only condition that must be met for a successful collision
how do catalysts affect the rate of reaction and why
provide an alternate reaction pathway
they
RA
reactants: bind to the reactants
activation: lower activation energy
proportion: increase proportion of successful collisions
what are the 5 things that affect the overall rate of a chemical reaction
TSP CC
temp
surface area (if solid)
pressure (if gaseous)
concentration (if solutions)
catalysts
Why do stronger bonds require more activation energy to be broken
because they are more stable, thus requiring more energy to be broken
what is the Maxwell-Boltzmann distribution curve and what can it be used to predict
describes the kinetic energy of particles of an ideal gas at a fixed temp
can be used to predict the:
PAP TC
probable: number of particles with the most probable kinetic energy
average kinetic energy of particles
proportion of particles with energy greater than the activation energy
temp: effect of changing temperature
catalyst: effect of adding a catalyst
what are energy profile diagrams and what can they be used to predict
similar to enthalpy change level diagrams
used to predict:
EA BE CI
exothermic: whether a reaction is exothermic or endothermic
activation: the activation energy for the forward and backward reaction
break: energy required to break or make bonds
enthalpy: overall enthalpy change
catalyst: effect of adding a catalyst
intermediate state
rate of reaction formula

how can a rate of reaction be measured
by observing changes in:
CVM
concentration
volume
mass