Types of Intermolecular Forces
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Intermolecular forces are forces between...
molecules
Molecules with an area of slight negative charge and an area of slight positive charge have a…
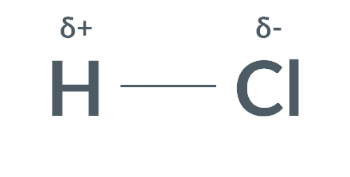
dipole
Permanent dipole-dipole forces are forces between…
molecules with partial charges
Does the following substance have permanent dipole-dipole forces between molecules?
H3O+
yes
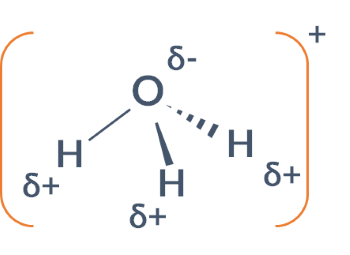
Does the following substance have permanent dipole-dipole forces between molecules?
NaCl
NaCl is an ionic compound which does not consist of molecules, only ionic bonds.
Therefore, NaCl cannot have permanent dipole-dipole interactions.
Explain how permanent dipole-dipole interactions arise between ICl molecules.
There is a difference in electronegativity between iodine and chlorine. This means that the ICl bond has a dipole and is polar. Permanent dipole-dipole interactions arise from the attraction between the δ+ side on one molecule and the δ− molecule on another
Explanation
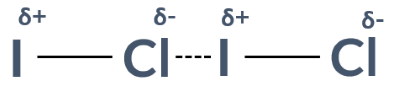
The strength of a dipole depends on…
the difference between the charges
Hydrogen bonding occurs when hydrogen is covalently bonded to…
N,O,F
Why does HF form hydrogen bonds whilst HBr does not?
F is more electronegative than Br
The movement of electrons in molecules means that nonpolar molecules can have…
temporary dipoles
How does London forces (also known as induced dipole-dipole forces) arise?
when the movement of electrons in one molecule creates a temporary dipole. This induces a temporary dipole in a nearby molecule.
This results in the attraction between partial charges of the nearby molecules
Which of the following experiences London forces?
Polar molecules.
Nonpolar molecules.
Elements that exist as single atoms
List the intermolecular forces that will exist between molecules of…
London forces
Hydrogen bonds
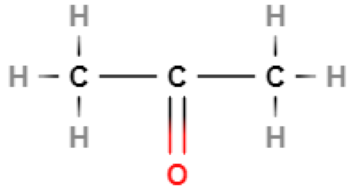
List the intermolecular forces that will exist between molecules of…
London forces
Permanent dipole-dipole forces
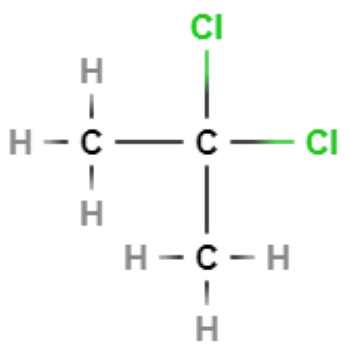
List the intermolecular forces that will exist between molecules of…
London forces
Permanent dipole-dipole forces
The strength of London forces increases as…
the number of electrons increases
induced dipole dipole force
This intermolecular force originates from temporary dipoles.
hydrogen bonds
This intermolecular force originates from N,O, or F atoms bonded to hydrogen
permanent dipole dipole forces
This intermolecular force originates from the asymmetric distribution of polar bonds
Explain how London forces arise between two molecules
The electrons in the first molecules move. When the electrons are unevenly distributed, a temporary dipole forms. This temporary dipole induces a dipole in the next molecule. The opposite charges of each dipole then attract each other.
Why are there no permanent dipole-dipole forces between SF6 molecules?
The dipoles cancel out
Which of the following hold the carbon and oxygen atoms together in a molecule of CO2?
Covalent bonding
Which of the following hold all the CO2 molecules together in sample of dry ice (solid CO2) ?
London forces
Intermolecular forces exist between molecules.
Oxygen is more electronegative than carbon so the C=O bond is polar and has a dipole.
However, the polar bonds are distributed symmetrically and so, they cancel out. This means that the CO2 molecule doesn’t have a dipole.
Therefore, only London forces can exist between CO2 molecules.
When a liquid is boiled, the molecules can move even further apart. So, the intermolecular forces have...
become weaker
How do you predict the strength of molecules with just london forces?
If the substances only have London forces, the molecule with the most electrons will have the highest melting and boiling points
Which molecule has the highest melting point?
Phosphorous (P4)
Sulphur (S8)
Chlorine (Cl2)
Argon (Ar)
All the molecules experience London forces.
S8 has the most electrons so it will experience the strongest London.
Therefore, S8 has the highest melting point
Can molecules with intermolecular forces conduct electricity…
never conduct electricity
The general rule of thumb to predict solubility is…
polar molecules dissolve in polar substances
nonpolar molecules dissolve in nonpolar substances
Why is ice less dense than water?
in ice, the hydrogen bonds hold the water molecules further away from each other. This results in large gaps in the lattice, and we call this structure an open lattice structure.
Now, this open lattice structure means that if we have an ice cube and a glass of water with the same number of molecules (and so, the same mass), the ice cube will occupy a larger volume of space. This difference in volume is why ice is less dense than water.
Why is this important?
If ice was denser than liquid water, it wouldn’t be able to float on top of it. This would mean that ice would sink to the bottom of all bodies of water, like lakes, rivers and oceans. This would freeze them from the bottom up, and would make it impossible for some underwater life to survive in them!
Why is CBr4 highly insoluble in water?
Bromine is more electronegative than carbon, so the C-Br bond has a dipole and is polar. However,CBr4 has tetrahedral geometry, and so the dipoles cancel out. Therefore, CBr4 is a nonpolar molecule. This means that it cannot form hydrogen bonds with water molecules, and so, is highly insoluble
Explain why ice floats on water
In ice, water molecules are held together in an open lattice structure. This means that the water molecules are held further apart in ice than in liquid water. This causes ice to be less dense than water and this is why ice can float on water