Cell Biology (Notes 32)
1/19
Earn XP
Description and Tags
Final Exam
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
How was the regulation of the cell cycle defined?
Budding Yeast Mutants of the Hartwell experiment: defined the “how” of the cell cycle
Mammalian Heterokaryons (cells with two distinct nuclei) from Rao and Johnson: observations made about the cell cycle
Fission Yeast Mutants from Nurse
Frog Extracts from Lohka and Matsui: identified proteins INVOLVED in the cell cycle
How does cdc28 mutant define start as a budding yeast?
Procedure of cloning by complementation discovered that the DNA in budding yeast was mutated —> cdc28
People discovered that cdc28 encoded a kinase - something is going to be phosphorylated
That’s what happened with Hartwell’s budding yeast story
What are defects in cell cycle progression caused by mutations in fission yeast (S. pombe)?
Evolutionarily, its distance from budding yeast isn’t great as the evolutionary distance between budding yeast and us
Paul Nurse mutagenized the cells and he discovered various mutants that exhibited defects in cell cycle progression. These mutants highlighted key checkpoints and regulatory mechanisms essential for proper cell division and function
These were cdc mutants (cell division control mutants)
Mutations:
In a cell that is wild type: yeast cell divides right down its center
In a cell with a recessive mutation: yeast cell become very large and doesn’t undergo cell division
In a cell with a dominant mutation: yeast cell are so small when they divide - never increase in length and then undergo cell division
Wee mutants
Found that the cdc2 gene, which is defective, encodes the fission yeast homologue of CDC28
Essentially, CDC28 and cdc2 are the same gene
However, they were discovered in a different order in different yeast - one got 28, the other got 2
One Master Kinase
Budding yeast, cdc28, S. pombe, cdc2, humans, cdc2, and frogs, MPF
There are three evolutionarily diverse organism and it’s all the same gene
Back in the early 80s, the mindset of biologists is that yeast are so evolutionarily distant from us that a yeast would look like a person
This discovery of the master kinase showed that what’s happening in a yeast cell is what’s happening in us at the cellular level
The core mechanistic level is that the organisms are pretty similar —> opened up the idea that organisms with lower complexity, like yeast, could be used to understand fundamental processes in humans
People started looking at fruit flies, worms, and the list goes on
When you see MPF, the same master kinase that’s in yeast mutants can be found in frogs
Fusion of an M-phase Cell with a G1-phase Cell Causes Condensation of the G1 Chromosomes
Researchers took a G1-phase cell and fused it with a mitotic cell - a classic, cell-cell fusion experiment
Once the cells fuse, the DNA in the G1 nucleus rapidly condensed
DNA synthesis did not occur - DNA went to G1 state, decondensed, to a mitotic cell
This is the EXCEPTION to the rule
Cells do not skip phases of the cell cycle - that’s the rule!
This experiment shows you can skip a phase
This is suggesting that there is something within the mitotic cell that is triggering and promoting the condensation of the G1 chromosome
So, what was the experiment?
Is there a diffusible cytoplasmic factor that pulls interphase cell into M phase?
Arrest the M phase cell
Transfer some of the M phase cytoplasm into a G2-phase arrested cell
The G2-phase arrested cell suddenly enters M phase
Just by transferring a little bit of the cytoplasm, you can change the cell
What promotes the pull of G2 to M phase?
There is the activity of the MPF = M-phase Promoting Factor
Not sure of the structure
The result is that cells are moving into M phase
How did they discover the MPF?
If you took cytoplasm from meiotic cells at different phases (G2 arrest, meiosis I, meiosis II), there’s a lot of MPF activity
That is the MATURATION PROMOTING FACTOR
At the same time, they were also looking at the early divisions of the frog embryonic mitosis —> that cytoplasm triggered the progression of the G2 cell into the M phase
There are ups and downs in the life of the cell where there was a factor present that promoted M phase
Maturation Promoting Factor = Mitosis Promoting Factor
What causes MPF activity to increase and decrease?
Frog egg was fertilized with a sperm
The zygotę will undergo mitotic divisions to form an embryo
Used cyclahexamide - protein synthesis is being inhibited
Fertilized the egg - nothing happened
In order for M phase to occur, there must be protein synthesis
Took same protein synthesis inhibited cells
Microinjected cytoplasm of a cell already in mitosis (MPF)
Those cells are still competent to undergo mitosis - by adding the cyclohexamide, it shows the protein synthesis is not inhibited
Can still get M phase to occur
Sea Urchin Embryos Contain Cyclin - A Protein That Increases and Decreases in Amount During the Cell Cycle
At different points post fertilization, researcher collected embryos and prepared them to be placed on an SDS Page gel
SDS Page: where you put a mixture of proteins on a gel matrix
Separate them on a basis of molecular weight
Minutes post fertilization, the SDS Page gel showed that some of the proteins are present over time
What the researcher noticed is that at cyclin at 76 minutes, the protein increases in abundance and then disappears completely
How do we put that result in a larger context?
If you look at the black line, that is the percentage of the cells dividing
There’s no divisions occurring until 65 minutes and then a line increase shows embryos going through their first mitotic divisions
Then, that number drops, then the embryos go through a second round of divisions (division, resting, division)
If we follow the darker red line, that is the intensity of the cyclin
Cyclin levels always peaked just prior to the onset of mitosis
As mitosis was starting up in the population of embryos, cyclin levels began to decrease
If we look at this protein, it cycles and is called cyclin
Cyclin is a component of MPF
What is MPF?
Kinase + cyclin that composes that factor
Hunt, in his famous paper, said that it is difficult to believe that the behavior of the cyclins is not connected with processes involved in cell division, but at this stage, we have no direct evidence that it is
Before the onset of the mitosis, he noticed cyclin levels going up but there was not a direct link between the two at the time, only a correlation
What is shown throughout the cell cycle?
MPF = [Kinase] Constant + [Cyclin] Variable
Aka cyclin-dependent kinase (Cdk)
Its concentration remains constant through a cell cycle - what is variable through a cell cycle is the abundance of the cyclin
One part of the MPF is constant (kinase), the other part is variable (cyclin)
What is the name of the kinase?
Cyclin-dependent kinase (Cdk)
Because it needs cyclin
How do we get to the cause and the effect between cyclin levels and mitosis?
Looking at the process of mitosis in the complete absence of a cell
Took fertilized frog embryos, crushed them, isolated the cytoplasm, then dropped it on the glass slide
Under certain inducing conditions, they can trigger the formation of mitotic spindles
From an experimental viewpoint, this was important with this in vitro system, we can easily add and subtract molecules
Can see what effect those molecules have on mitosis
Entry Into Mitosis Requires Cyclin Synthesis; Exit Requires Cyclin Degradation
Looking to see whether or not there’s an increase in MPF activity and an increase/decrease in protein cyclin levels
A.
Untreated Extract - no manipulations done to it
Get to M phase by adding sperm DNA
Over time, MPF activity will increase and then it will decrease
C.
RNAse-treated extract + wild-type cyclin B mRNA
If you track cyclin, the correlation is even tighter than the untreated extract
Blue rectangle = pre-metaphase
Orange rectangle = post-metaphase (anaphase)
MPF activity increasing with increasing cyclin levels
Plunge in anaphase
repeat
Another round of M phase
What’s important for M phase to occur is protein synthesis
B.
RNase-treated extract
If you have no mRNA, you can’t have protein synthesis
Treat the extracts with RNase, nothing happens (with the addition of sperm nuclei)
The level of MPF activity is flat… the level of cyclin concentration is flat …
D.
RNase-treated extract + non degradable cyclin B mRNA
Add back to the mRNA for non degradable cyclin B
It’s translated in the extract - start the process by adding sperm nuclei
Get the MPF activity and cyclin B just like the untreated extract
Without degradation, cyclin can’t exit mitosis (mitotic arrest - stopped in this stage)
Have to get rid of cyclin B to lose the kinase
Not getting past metaphase (blue rectangle) - mutations
What is the degradation process to degrade cyclin?
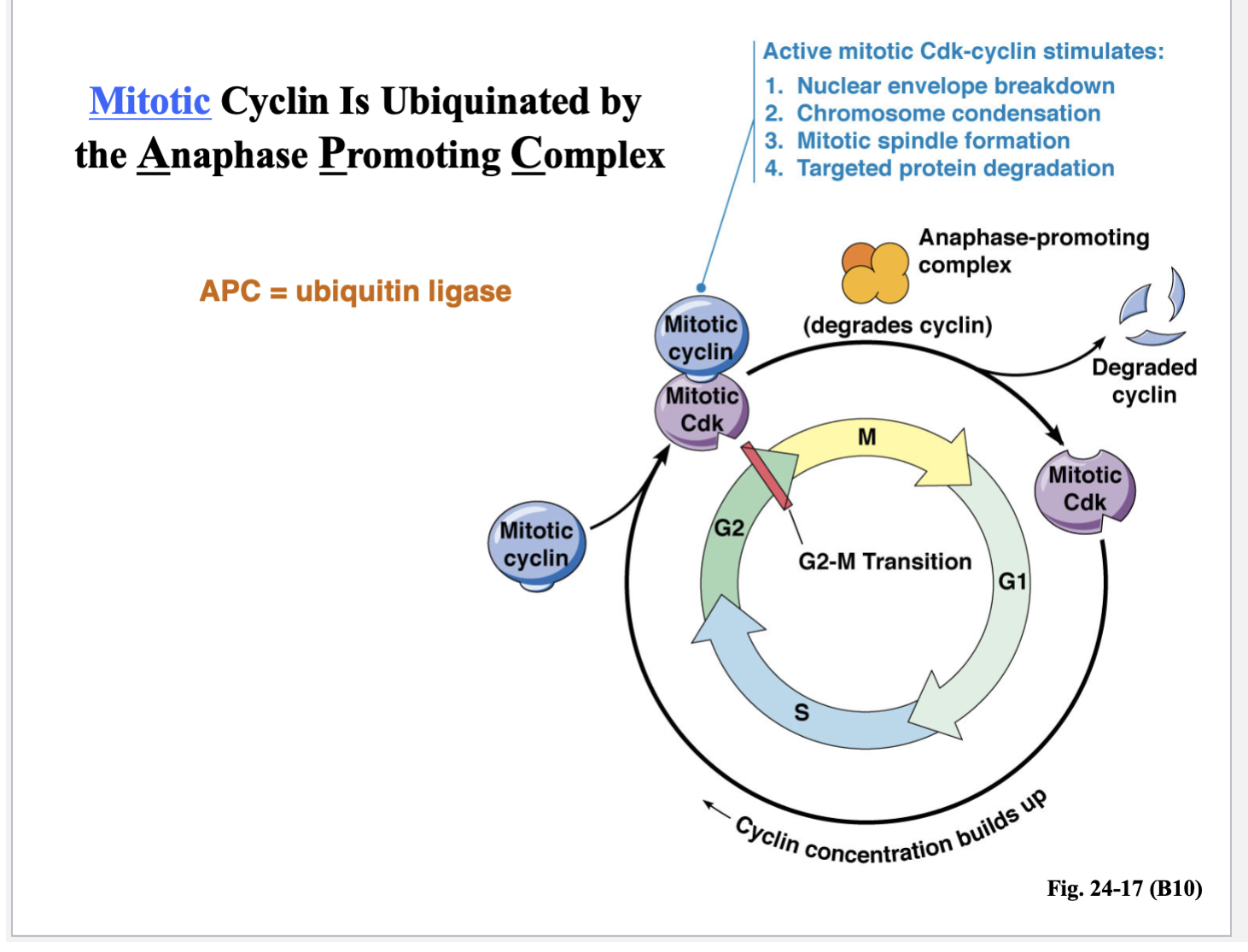
Mitotic cyclin is UBIQUINATED by the anaphase promoting complex (APC = ubiquitin ligase)
This is a protein complex - an enzyme that adds the ubiquitin to the target protein or the cyclin
Its job is to degrade cyclin so that the cell can move into anaphase and exit mitosis or M phase
Active mitotic Cdk-cyclin stimulates:
Nuclear envelope breakdown
Chromosome condensation
Mitotic spindle formation
Targeted protein degradation
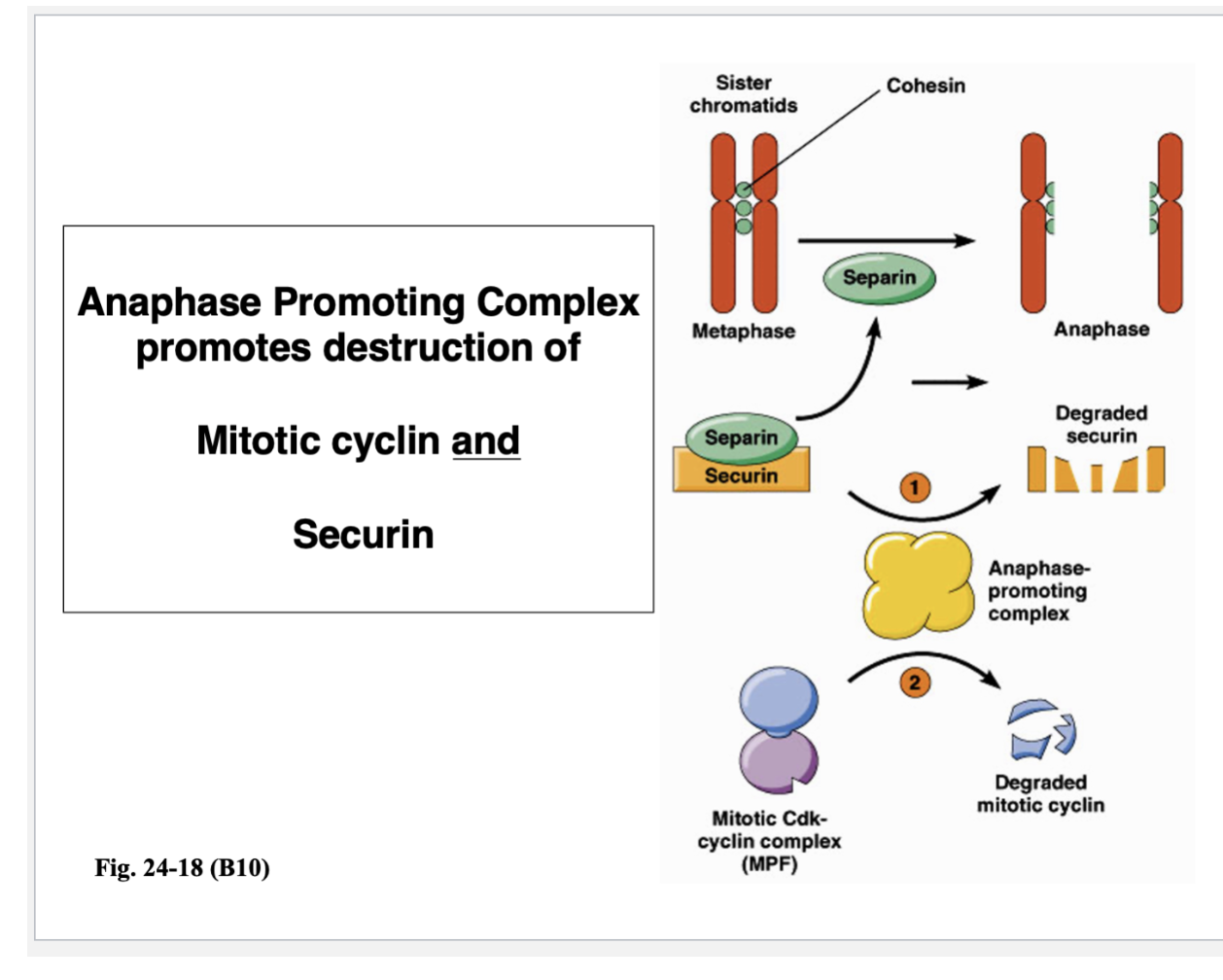
Anaphase Promoting Complex promotes destruction of …
Mitotic cyclin
Securin
Anaphase promoting complex is necessary for allowing sister chromatids to separate from each other
In anaphase A, sister chromatids come apart and move towards the spindle poles
Before those motors can drag those sister chromatids, those sister chromatids have to be released from each other, which are held together by protein cross-links
Those protein cross-links are present - can’t pull sister chromatids apart
APC does this, but not directly
To break a protein cross-link, the protein that destroys the cohesion cross-links is separin
Separin destroys those cohesin cross-links — early phase of mitosis (pre-metaphase)
You don’t want them coming apart too early
Separin can’t act on cohesin because it is held by securin
APC that degrades securin
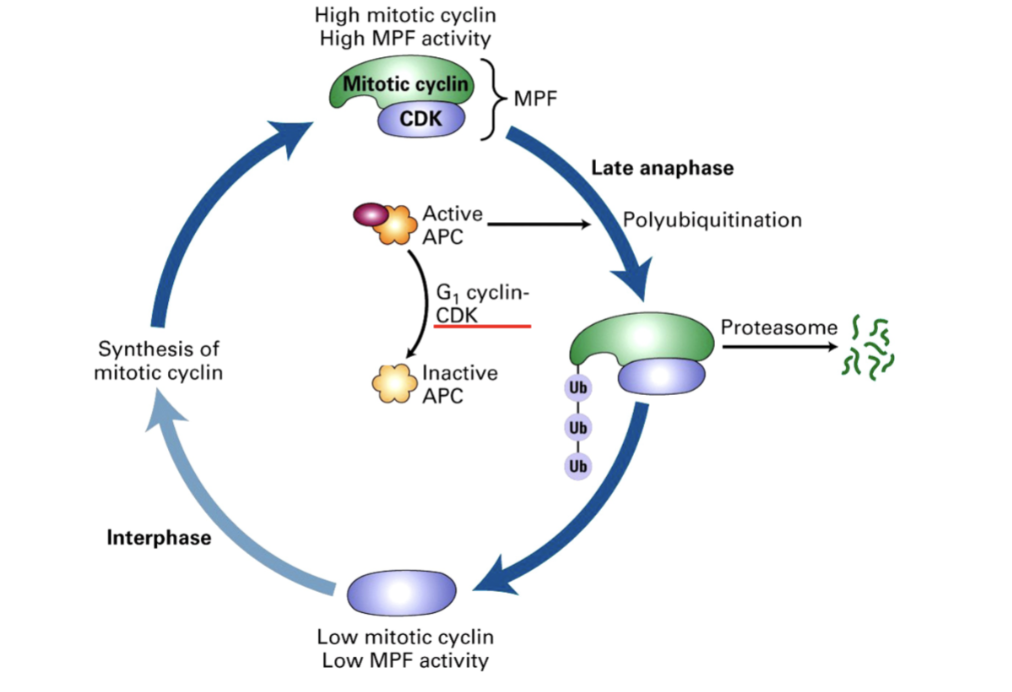
How do we get out of M phase and back into G1?
APC has to be inactivated by phosphorylation event controlled by cyclin-dependent kinase
This Cdk is not associated with mitotic cyclin but a G1 cyclin
G1 Cyclin
CDK Complex that inactivates APC
Allows cell to move from M phase to G1
By promoting degradation of mitotic cyclins
How many cyclins and cyclin-dependent kinases (CDKs) exist?
Different parts of the cell cycle have multiple CDKs/cyclins in mammals
One CDK, multiple cyclins in yeast
Each peak in different parts of the cell
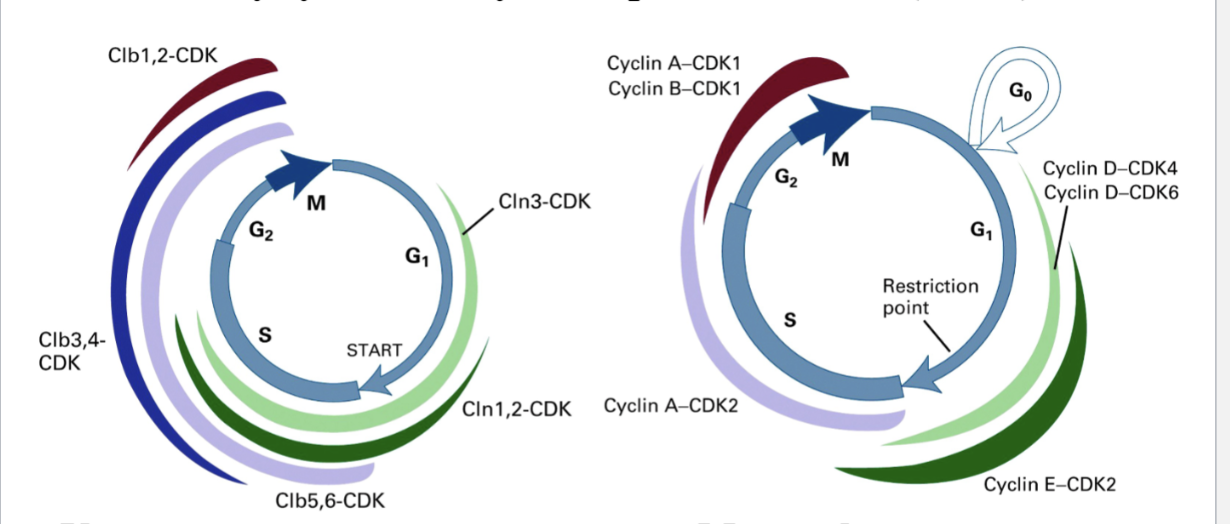
What are the four classes of cyclin that activate CDKs in specific phases of the cell cycle?
M-phase Cyclins (e.g., Cyclin B) promote mitosis
G1-phase Cyclins promote passage thru START or R-point
G1/S-phase Cyclins commit the cell to DNA synthesis
S-phase Cyclins promote the initiation of DNA synthesis
Cyclins are necessary for activation of CDKs and allow CDKs to phosphorylate specific substrates
M phase cyclin-CDK will phosphorylate different proteins than G1 cyclin-CDK
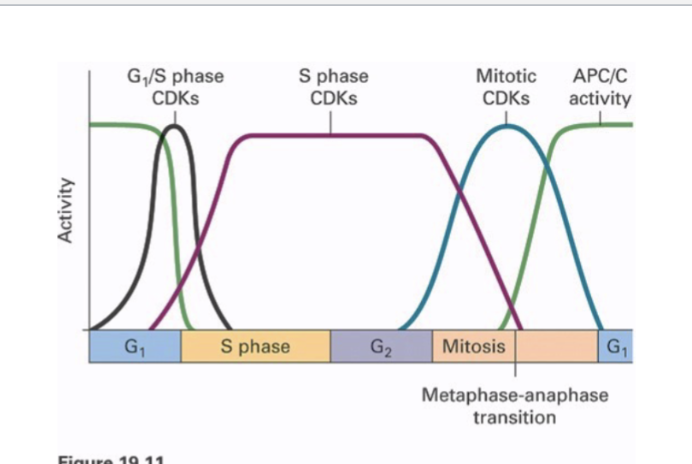
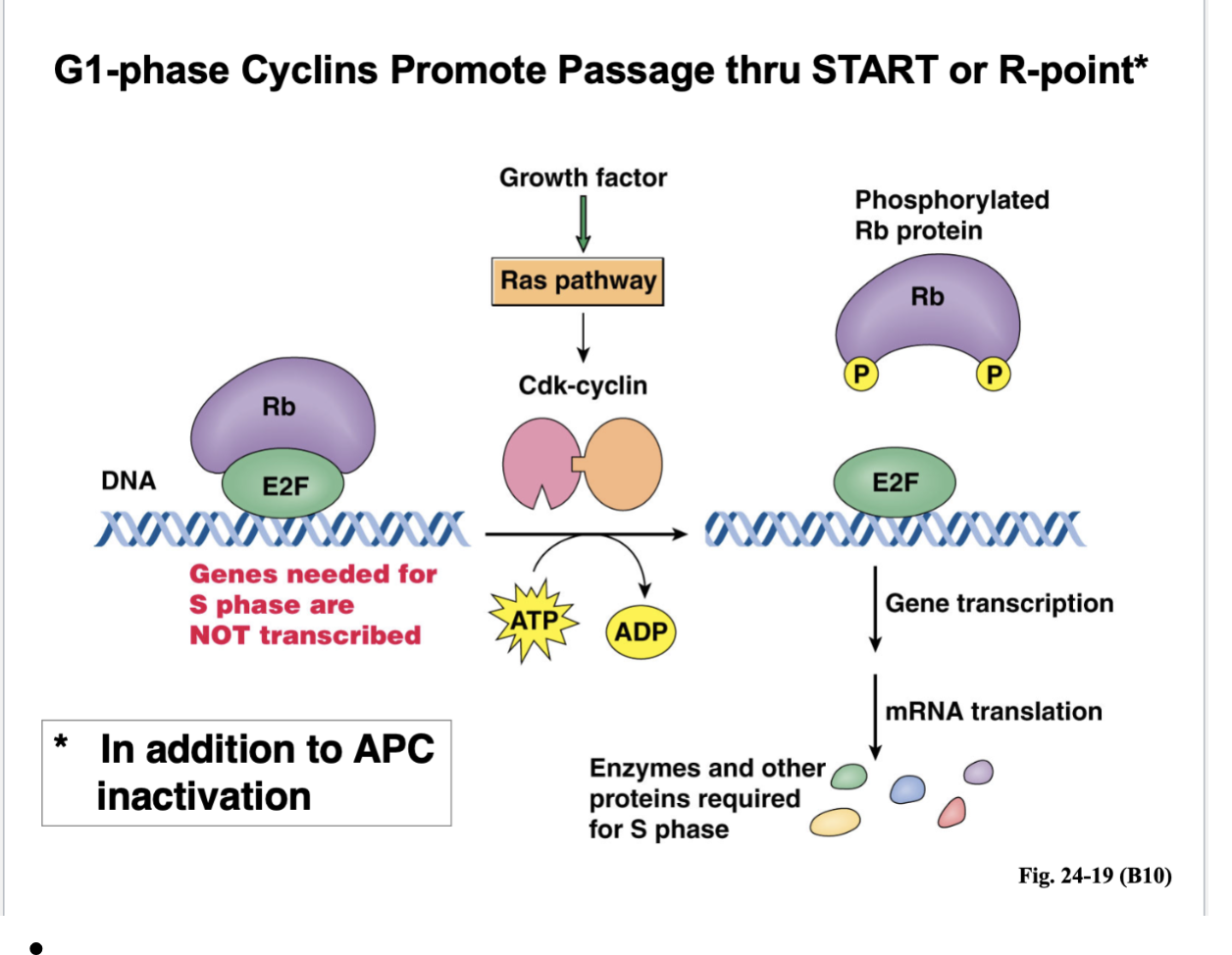
G1-phase Cyclins Promote Passage thru START or R-point*
Shortly after START, there’s DNA replication
How are you passing START and entering the next cell cycle?
You need S phase where DNA replication occurs
Right in G1, the genes needed for S phase are NOT transcribed
THere’s a transcription factor called E2F because it is bound to Rb (retinoblastoma) protein
What would happen is when you’re passing the R point, the growth factors is activating a Ras signaling pathway
That Ras pathway, through a whole series of steps leads to the formation of Cdk-cyclin
Those are G1 phase cyclin — that Cdk is now active and what that kinase does is phosphorylate the Rb protein
When Rb is phosphorylated, it can no longer bind to the E2F transcription factor
E2F is free to play the role in gene transcription, mRNA translation, and enzymes and other proteins required for S phase