Intermediate Filaments & Septins
0.0(0)
0.0(0)
Card Sorting
1/17
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
1
New cards
Intermediate Filaments (IFs)
\
* are strong rope-like polymers of fibrous proteins that resist stretch
* Give mechanical integrity similar to a rope (fibers together are strong)
* IFs share 5 basic characteristics
* 1. Are \~10 nm in diameter (intermediate between actin thin filaments and myosin thick filaments).
* 2. Are very stable – disassembly in vitro requires treatment with agents that denature proteins.
* 3. Do not bind nucleotides.
* 4. Are non-polar in structure.
* 5. Can withstand stretching forces.
* Intermediate filaments play a structural or tension-bearing role in the cell and are important for cell strength and cell-cell attachments.
* are strong rope-like polymers of fibrous proteins that resist stretch
* Give mechanical integrity similar to a rope (fibers together are strong)
* IFs share 5 basic characteristics
* 1. Are \~10 nm in diameter (intermediate between actin thin filaments and myosin thick filaments).
* 2. Are very stable – disassembly in vitro requires treatment with agents that denature proteins.
* 3. Do not bind nucleotides.
* 4. Are non-polar in structure.
* 5. Can withstand stretching forces.
* Intermediate filaments play a structural or tension-bearing role in the cell and are important for cell strength and cell-cell attachments.
2
New cards
Intermediate Filament structure
\
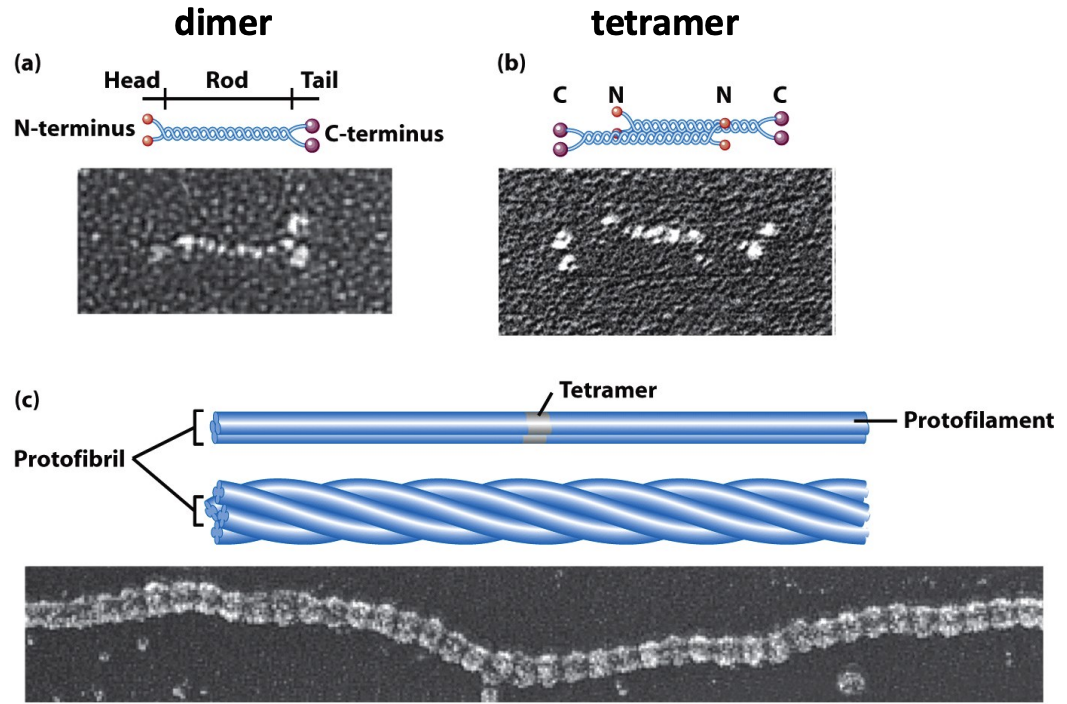
* IF proteins have a central αhelical coiled-coil rod domain that is highly conserved and heads and tails that are variable in length and sequence.
* They form a parallel dimer that assembles into an antiparallel tetramer. Tetramers assemble end-to end to form protofilaments, which associate laterally to form protofibrils.
* Protofibrils further assemble into an IF.
* Because the tetrameric subunit has no polarity, the filament has no polarity
* IF proteins have a central αhelical coiled-coil rod domain that is highly conserved and heads and tails that are variable in length and sequence.
* They form a parallel dimer that assembles into an antiparallel tetramer. Tetramers assemble end-to end to form protofilaments, which associate laterally to form protofibrils.
* Protofibrils further assemble into an IF.
* Because the tetrameric subunit has no polarity, the filament has no polarity
3
New cards
Assembly of IFs in cells
\
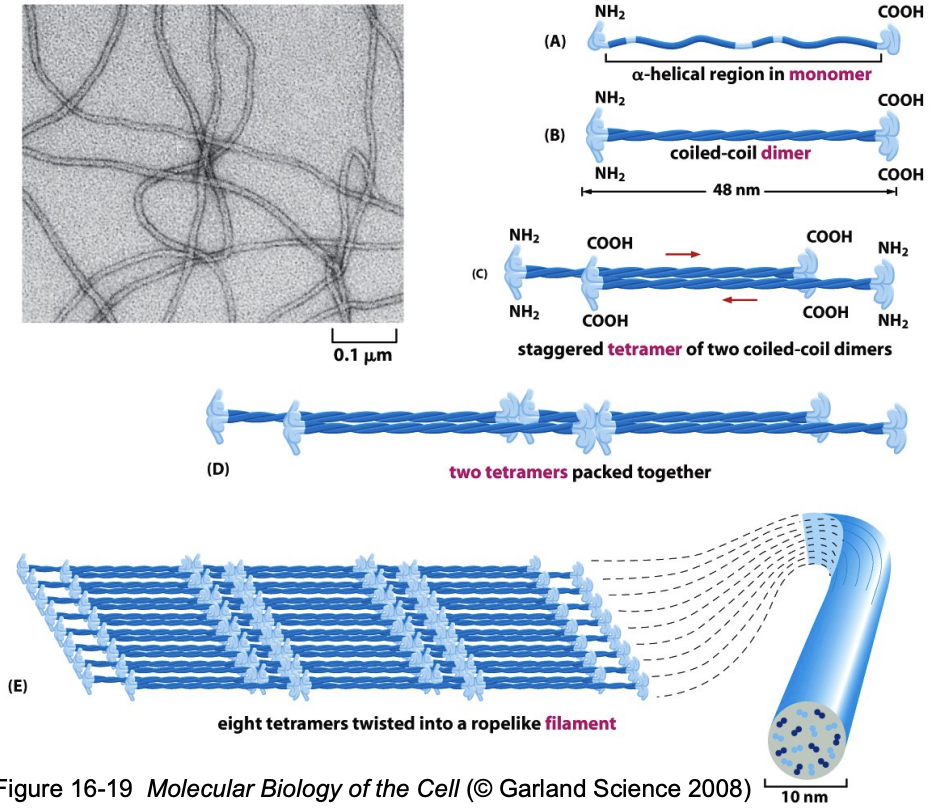
* Soluble tetramers can be isolated from cells, suggesting that this is the basic building block, or functional subunit, of the filament.
* The soluble pool of IF tetramer in the cell is very small: 1-5% of the total intermediate filament protein. (In contrast, \~40% of actin is present as monomer subunits.)
* There appears to be a direct, random exchange between the pool of IF subunits and those in the insoluble filaments in the cell; the dynamics of these filaments are very modest compared to actin filaments and microtubules.
* Soluble tetramers can be isolated from cells, suggesting that this is the basic building block, or functional subunit, of the filament.
* The soluble pool of IF tetramer in the cell is very small: 1-5% of the total intermediate filament protein. (In contrast, \~40% of actin is present as monomer subunits.)
* There appears to be a direct, random exchange between the pool of IF subunits and those in the insoluble filaments in the cell; the dynamics of these filaments are very modest compared to actin filaments and microtubules.
4
New cards
Intermediate Filament incorporation assay:
\
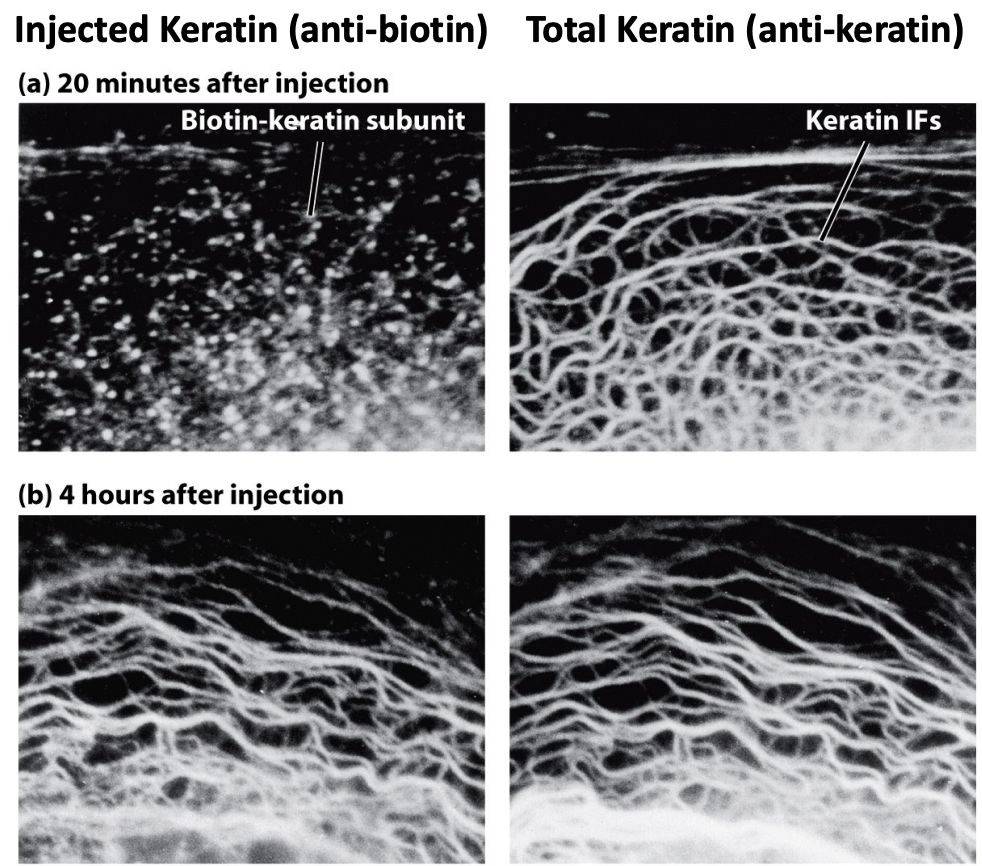
* Keratins incorporate throughout the filaments
* Instead of growing at the ends, they randomly incorporate slowly
* Over time, the keratin will incorporate into existing skeleton
* Right after injection they don't incorporate yet
* Keratins incorporate throughout the filaments
* Instead of growing at the ends, they randomly incorporate slowly
* Over time, the keratin will incorporate into existing skeleton
* Right after injection they don't incorporate yet
5
New cards
Microtubule incorporation assay:
\
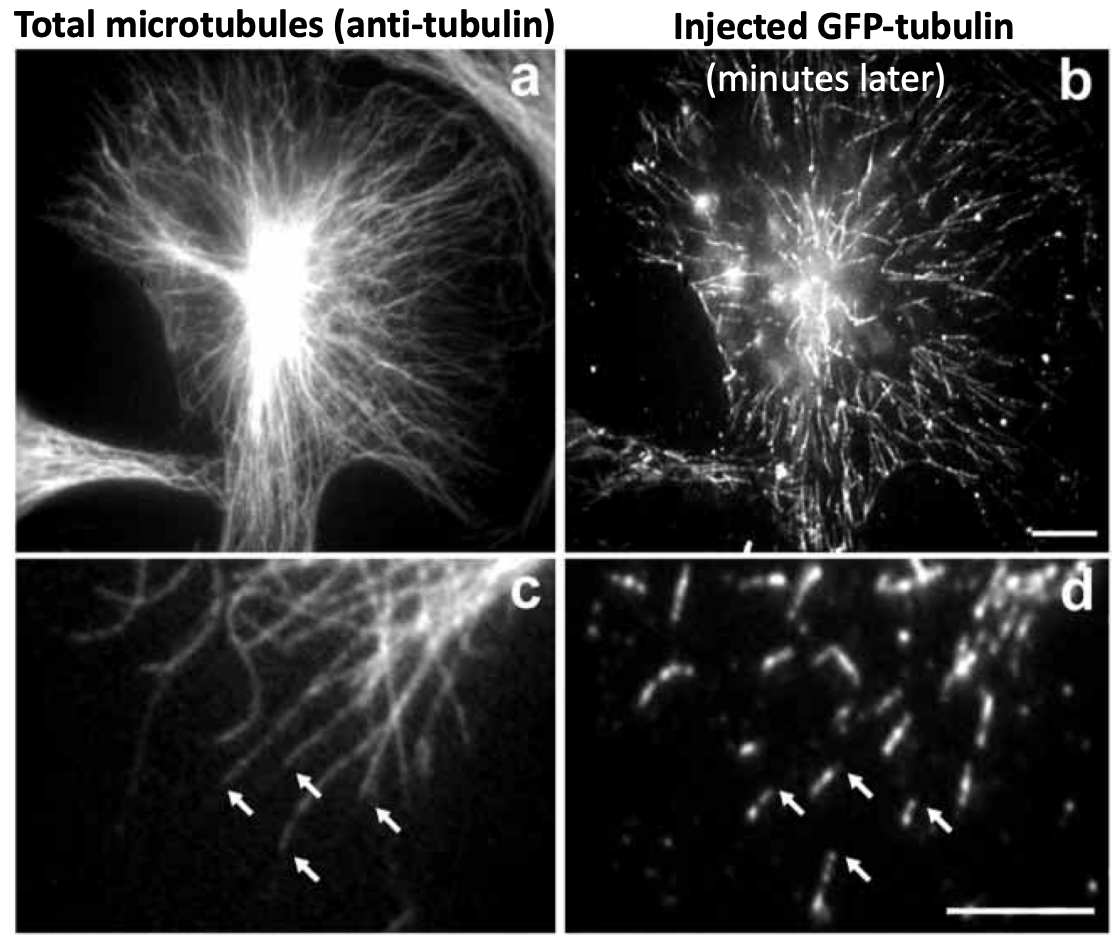
* Tubulin subunits incorporate at growing + ends
* This is different from IF
* Incorporate faster at + ends
* Tubulin subunits incorporate at growing + ends
* This is different from IF
* Incorporate faster at + ends
6
New cards
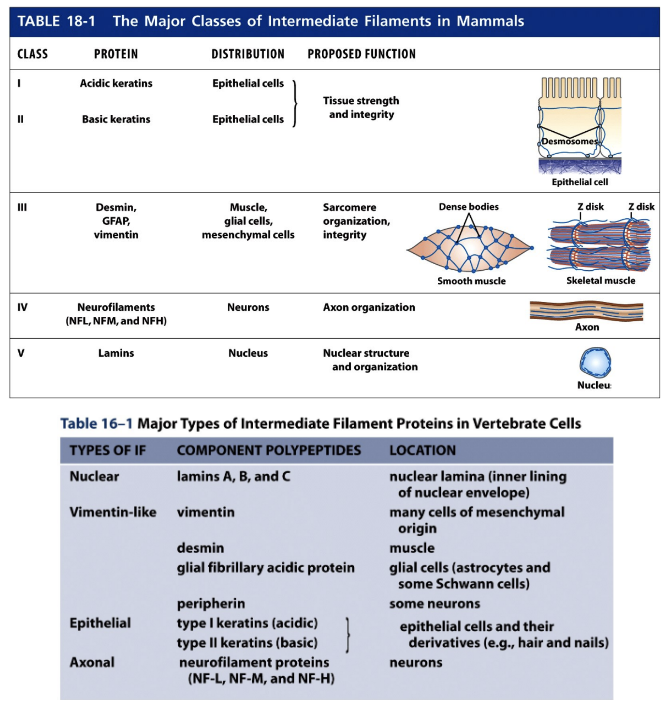
Types of Intermediate Filaments
Type I (acidic) and Type II (basic) Keratins
\
Type III Vimentin and Vimentin-related filaments
\
Type IV Neurofilaments
\
Nuclear Lamins
\
Type III Vimentin and Vimentin-related filaments
\
Type IV Neurofilaments
\
Nuclear Lamins
7
New cards
Type I (acidic) and Type II (basic) Keratins
\
* These form heteropolymers composed of acidic and basic subunits. There are over 20 keratins expressed in human epithelial cells. Keratins are found in epithelia that line internal body cavities. Because different cell types express different sets of keratins, the origin of epithelial cancers (carcinomas) can be determined based on the set of keratins found in the tumor cells. There are also about 10 ‘hard’ keratins that are found in hair and nails. Keratins in the outermost layer of the skin become cross-linked together, and as cells die the keratins persist as a protective layer.
* These form heteropolymers composed of acidic and basic subunits. There are over 20 keratins expressed in human epithelial cells. Keratins are found in epithelia that line internal body cavities. Because different cell types express different sets of keratins, the origin of epithelial cancers (carcinomas) can be determined based on the set of keratins found in the tumor cells. There are also about 10 ‘hard’ keratins that are found in hair and nails. Keratins in the outermost layer of the skin become cross-linked together, and as cells die the keratins persist as a protective layer.
8
New cards
Type III Vimentin and Vimentin-related filaments
These proteins can form homopolymers that consist of a single IF protein species. Vimentin is the most widely distributed intermediate filament protein. It is found in fibroblasts, endothelial cells, white blood cells, and embryonic cells. Another type III protein, desmin, is found mainly in muscle cells where it links adjacent Zdisks of myofibrils. Glial fibrillary acidic protein (GFAP) is a vimentin-related protein found in the glial cells that surround neurons and astrocytes.
9
New cards
Type IV Neurofilaments
These are composed of heteropolymers of NF-L, NF-M, and NF-H. They are found in axons and are important for providing strength in the axon and determining its diameter
10
New cards
Nuclear Lamins
\
* The nuclear lamina is a meshwork of IFs that lines the inner surface of the nuclear membrane. It is composed of lamins A, B and C. Nuclear lamins differ from other IF proteins in that they: have a longer central rod domain, contain a nuclear import signal, assemble into a two dimensional sheet-like lattice, and are more dynamic than other IFs. Disassembly of the nuclear lamina during mitosis is mediated by phosphorylation.
* The nuclear lamina is a meshwork of IFs that lines the inner surface of the nuclear membrane. It is composed of lamins A, B and C. Nuclear lamins differ from other IF proteins in that they: have a longer central rod domain, contain a nuclear import signal, assemble into a two dimensional sheet-like lattice, and are more dynamic than other IFs. Disassembly of the nuclear lamina during mitosis is mediated by phosphorylation.
11
New cards
Organization and Function of IFs
\
* Intermediate filament binding proteins (IFBPs) cross-link IFs into networks or bundles. The network of IFs in the cell is often coincident with the microtubule cytoskeleton. In some cell types, disruption of the microtubule cytoskeleton with microtubule de-stabilizing drugs causes the IF network to collapse.
* Nuclear positioning and support of the nuclear envelope.
* IFs such as vimentin typically extend from the nuclear envelope to the plasma membrane, perhaps helping to position the nucleus. The network of lamins at the inner surface of the nuclear membrane helps maintain the structure of the nucleus and is important for nuclear assembly and disassembly.
* Cell-cell attachments: desmosomes and hemi-desmosomes.
* The epithelia of organs and skin are held together by cell junctions called desmosomes and hemi-desmosomes. Desmosomes function in cell-cell interactions, whereas hemi-desmosomes are important for the interaction of cells with the extracellular matrix. Intermediate filaments run between desmosomes, hemidesmosomes and the nucleus. Together IFs and desmosomes provide each cell and the entire epithelium with strength and rigidity.
* Muscle cell integrity.
* Desmin links the Z-disks of skeletal muscle together within the myofibril. Moreover, it connects Z-disks to cell junctions in cardiac muscle.
* Intermediate filament binding proteins (IFBPs) cross-link IFs into networks or bundles. The network of IFs in the cell is often coincident with the microtubule cytoskeleton. In some cell types, disruption of the microtubule cytoskeleton with microtubule de-stabilizing drugs causes the IF network to collapse.
* Nuclear positioning and support of the nuclear envelope.
* IFs such as vimentin typically extend from the nuclear envelope to the plasma membrane, perhaps helping to position the nucleus. The network of lamins at the inner surface of the nuclear membrane helps maintain the structure of the nucleus and is important for nuclear assembly and disassembly.
* Cell-cell attachments: desmosomes and hemi-desmosomes.
* The epithelia of organs and skin are held together by cell junctions called desmosomes and hemi-desmosomes. Desmosomes function in cell-cell interactions, whereas hemi-desmosomes are important for the interaction of cells with the extracellular matrix. Intermediate filaments run between desmosomes, hemidesmosomes and the nucleus. Together IFs and desmosomes provide each cell and the entire epithelium with strength and rigidity.
* Muscle cell integrity.
* Desmin links the Z-disks of skeletal muscle together within the myofibril. Moreover, it connects Z-disks to cell junctions in cardiac muscle.
12
New cards
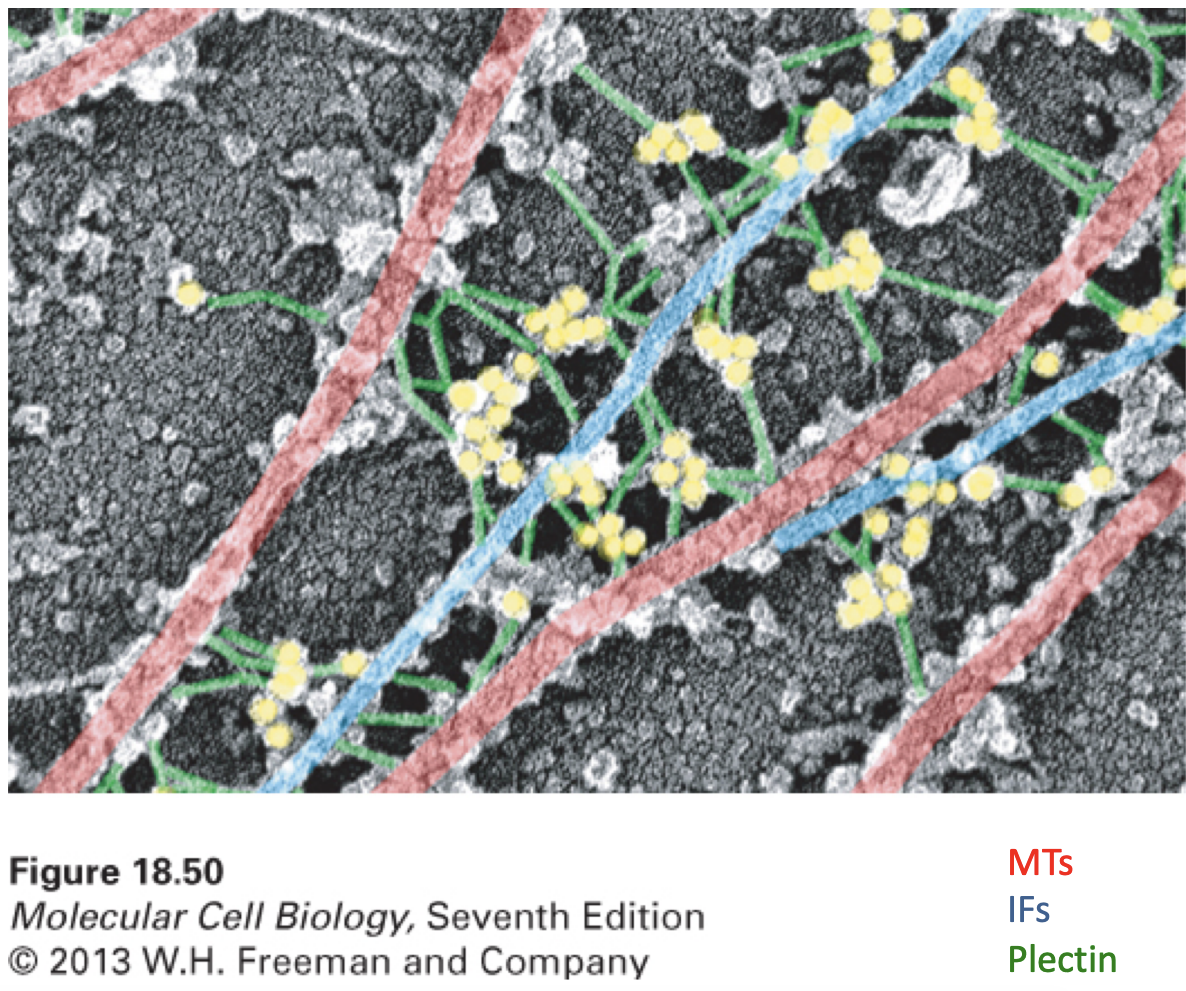
Crosslinking of MTs to IFs by Plectin
\
* There can be interactions between different cytoskeletal elements like MTs and IFs being linked by Plectin
* There can be interactions between different cytoskeletal elements like MTs and IFs being linked by Plectin
13
New cards
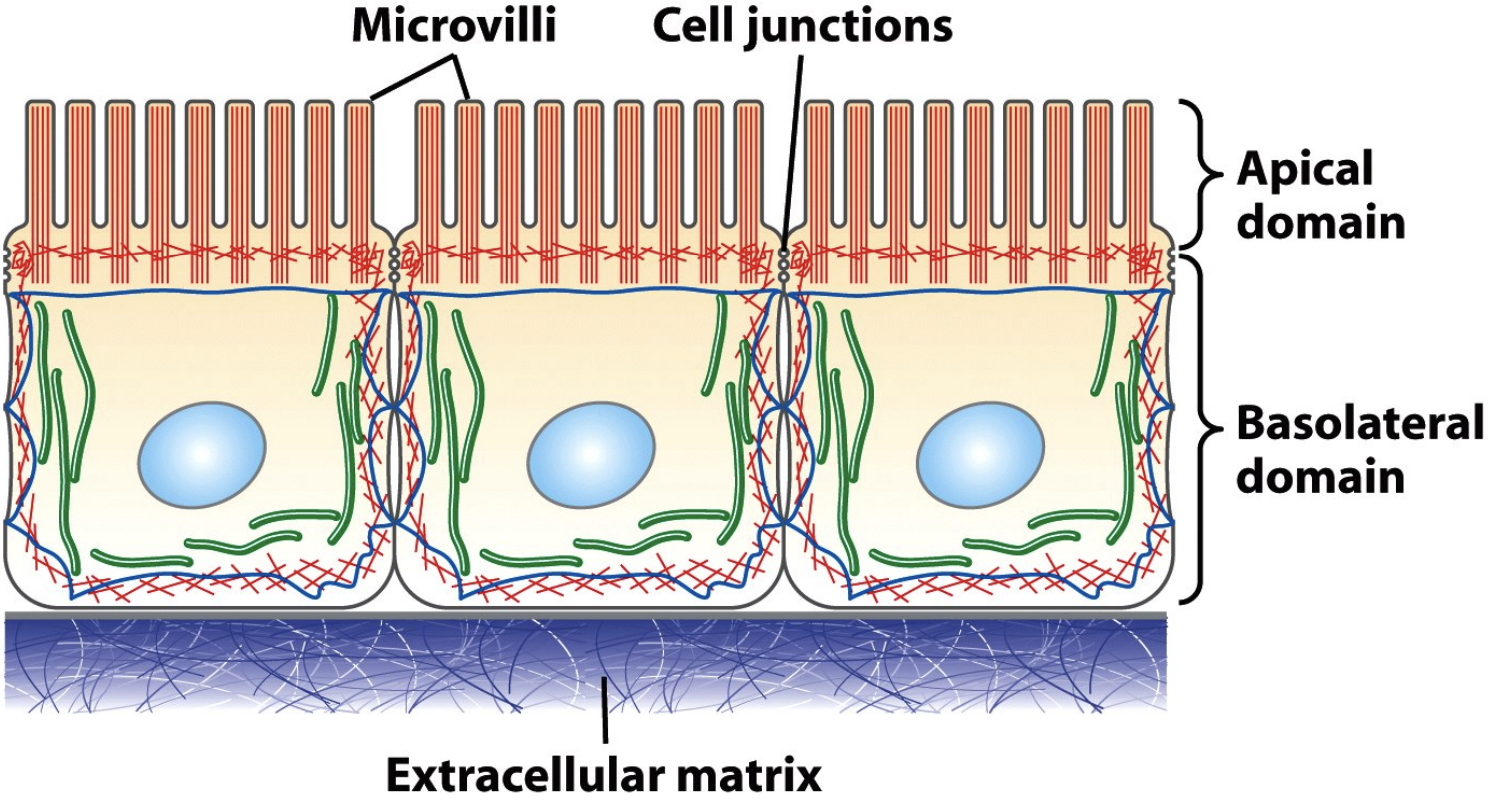
IFs at desmosomes and hemi-desmosomes
\
* Cell-cell adhesion
* 3 epithelial cells undergo adhesions with each other and with the matrix
* Desmosomes are a cell-cell interaction (the sides) and those adhesions are linked to IF inside the cells
* Hemi-desmosomes are connections between the bottom of the cell and the extracellular matrix, linked to IF in the cell
* Gives structural integrity by attaching to matrix and prevents cells being ripped apart
* Cell-cell adhesion
* 3 epithelial cells undergo adhesions with each other and with the matrix
* Desmosomes are a cell-cell interaction (the sides) and those adhesions are linked to IF inside the cells
* Hemi-desmosomes are connections between the bottom of the cell and the extracellular matrix, linked to IF in the cell
* Gives structural integrity by attaching to matrix and prevents cells being ripped apart
14
New cards
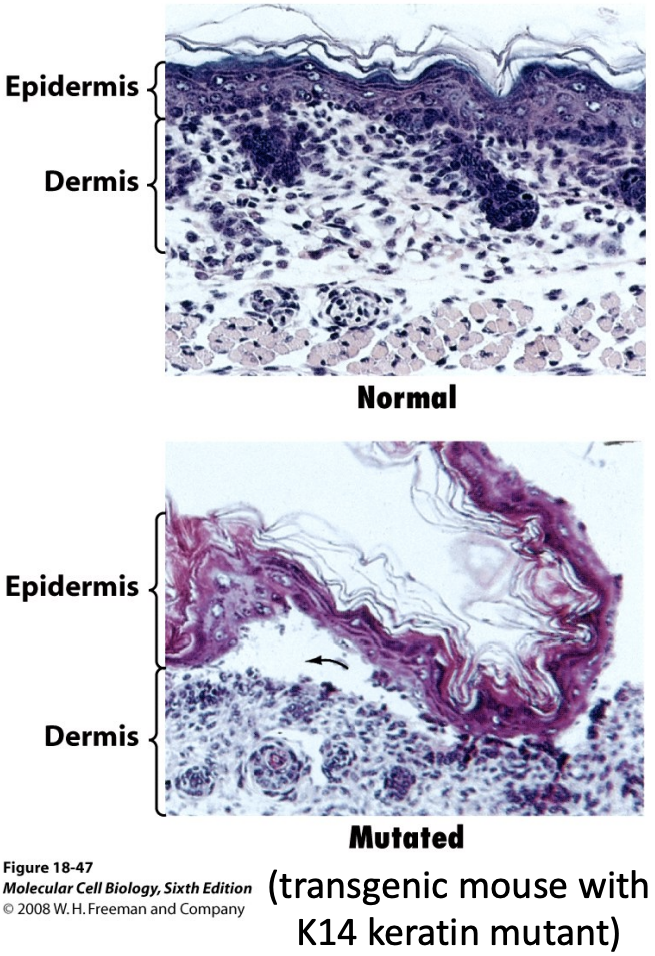
Intermediate Filaments and Disease
\
* Genes encoding IFs are associated with >40 clinical disorders.
* The genetic disease epidermolysis bullosa simplex (EBS) is caused by mutations in keratin genes expressed in skin. The skin of people with this disease is very sensitive to injury.
* More than 50 different mutations in type-A lamins cause disease, including Emerey-Dreifuss muscular dystrophy (EDMD), cardiomyopathy, and progeria (premature aging).
* Genes encoding IFs are associated with >40 clinical disorders.
* The genetic disease epidermolysis bullosa simplex (EBS) is caused by mutations in keratin genes expressed in skin. The skin of people with this disease is very sensitive to injury.
* More than 50 different mutations in type-A lamins cause disease, including Emerey-Dreifuss muscular dystrophy (EDMD), cardiomyopathy, and progeria (premature aging).
15
New cards
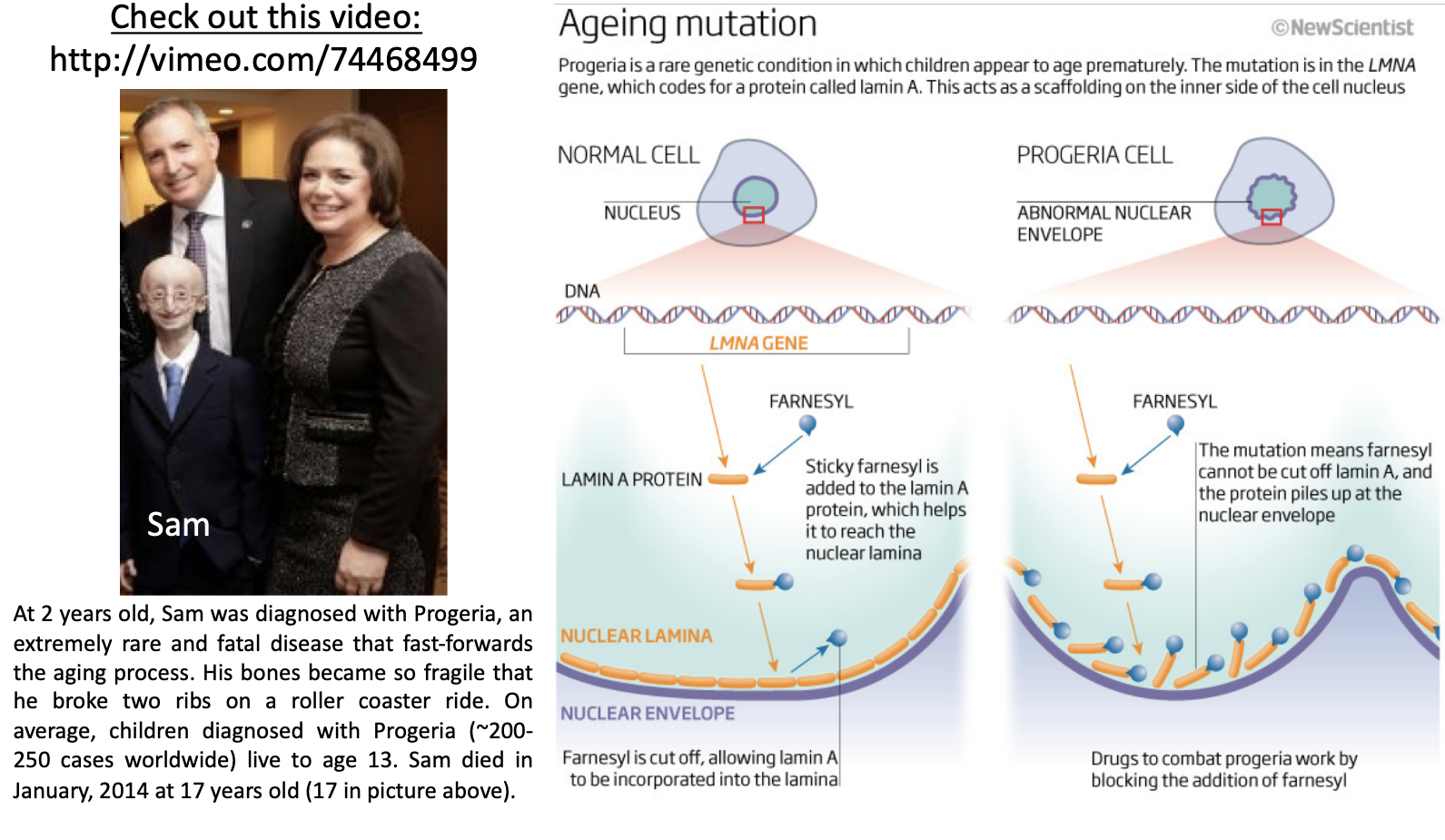
Hutchinson-Gilford Progeria Syndrome (Progeria)
\
* results from mutations in the lamin A gene
* Premature aging
* Mutations in lamin A lead to this
* In normal cells, lamins are farnesylated to get incorporated into lamina and unfarnesylated
* In progeria cells, lamins are farnesylated but can’t incorporate properly into lamina because can’t unfarnesylated
* Nuclear envelope is disfigured
* Telomere biology can’t happen causing again
* results from mutations in the lamin A gene
* Premature aging
* Mutations in lamin A lead to this
* In normal cells, lamins are farnesylated to get incorporated into lamina and unfarnesylated
* In progeria cells, lamins are farnesylated but can’t incorporate properly into lamina because can’t unfarnesylated
* Nuclear envelope is disfigured
* Telomere biology can’t happen causing again
16
New cards
septins
\
* An underappreciated 4th cytoskeletal element
* Septin filaments are 10nm in diameter, bind and hydrolyze GTP, and can be found associated with actin filaments and membranes.
* Non-olar subunits (hexamers or octamers), thus non-polar polymers
* Septins are members of a highly conserved protein family first identified in the yeast Saccharomyces cerevisiae (baker’s yeast) and more recently found in animal cells.
* Septins were originally identified in a screen for yeast mutants that arrest in various stages of the cell cycle (called cdc mutants for cell division cycle). Cells with mutations in the septin genes cdc3, cdc10, cdc11 and cdc12 all arrest at cytokinesis.
* Septins were later found to be the structural components of filaments observed at the neck between the mother and daughter yeast cell (the bud neck).
* In mammalian cells, septin filaments colocalize with F-actin in stress fibers and with F-actin in the contractile ring.
* An underappreciated 4th cytoskeletal element
* Septin filaments are 10nm in diameter, bind and hydrolyze GTP, and can be found associated with actin filaments and membranes.
* Non-olar subunits (hexamers or octamers), thus non-polar polymers
* Septins are members of a highly conserved protein family first identified in the yeast Saccharomyces cerevisiae (baker’s yeast) and more recently found in animal cells.
* Septins were originally identified in a screen for yeast mutants that arrest in various stages of the cell cycle (called cdc mutants for cell division cycle). Cells with mutations in the septin genes cdc3, cdc10, cdc11 and cdc12 all arrest at cytokinesis.
* Septins were later found to be the structural components of filaments observed at the neck between the mother and daughter yeast cell (the bud neck).
* In mammalian cells, septin filaments colocalize with F-actin in stress fibers and with F-actin in the contractile ring.
17
New cards
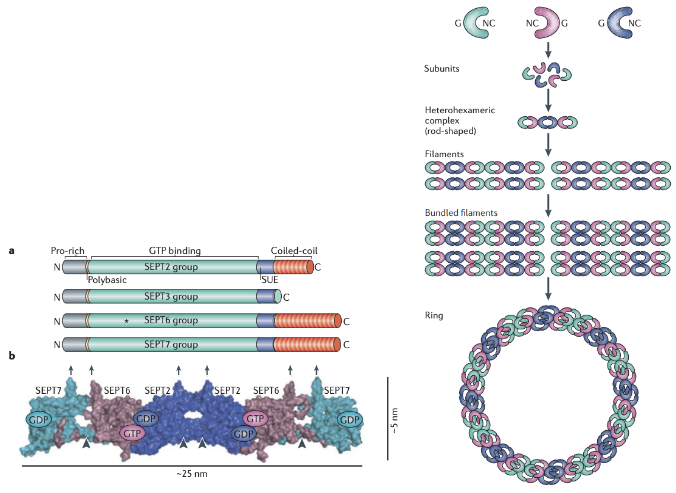
Organization of Septin filaments
\
* Individual septin proteins have a GTP-binding domain near their N-terminus and an a-helical coiled coil region at their C-terminus. The role of GTP hydrolysis in filament assembly is not yet known
* 2 NC domains interact, 2 G domains interact forming a hexamer or octomer
* Septins are thought to form nonpolar subunits of heterohexamers or heterooctamers that can further assemble into filaments, rings or cage-like structures (like contractile ring)
* Individual septin proteins have a GTP-binding domain near their N-terminus and an a-helical coiled coil region at their C-terminus. The role of GTP hydrolysis in filament assembly is not yet known
* 2 NC domains interact, 2 G domains interact forming a hexamer or octomer
* Septins are thought to form nonpolar subunits of heterohexamers or heterooctamers that can further assemble into filaments, rings or cage-like structures (like contractile ring)
18
New cards
Cellular Functions of Septin Filaments
\
* 1. Cytokinesis: In mammalian cells and in budding yeast, septins are found at the cleavage furrow and cytokinetic ring. Septin mutations in yeast cause cytokinesis to fail.
* 2. Scaffolding: Septins may play a role in recruiting signaling proteins such as protein kinases and GTPases that regulate cytokinesis and other aspects of cell function.
* 3. Diffusion Barrier: Septins can promote compartmentalization of cellular domains.
* 4. Membrane trafficking and dynamics: Septins may play a role in the movement and fusion of membrane vesicles and organelle remodeling.
\
\
* The actin and microtubule cytoskeletons drive mitosis and cytokinesis (but lamins and septins are important too)
* 1. Cytokinesis: In mammalian cells and in budding yeast, septins are found at the cleavage furrow and cytokinetic ring. Septin mutations in yeast cause cytokinesis to fail.
* 2. Scaffolding: Septins may play a role in recruiting signaling proteins such as protein kinases and GTPases that regulate cytokinesis and other aspects of cell function.
* 3. Diffusion Barrier: Septins can promote compartmentalization of cellular domains.
* 4. Membrane trafficking and dynamics: Septins may play a role in the movement and fusion of membrane vesicles and organelle remodeling.
\
\
* The actin and microtubule cytoskeletons drive mitosis and cytokinesis (but lamins and septins are important too)