Introduction to Materials Science and Engineering
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
Stone Age (2.6 million years ago – 3300 B.C.)
Bronze Age (3300 B.C. – 1200 B.C.)
Iron Age (1200 B.C – 600 B.C.)
Advanced Materials (early 20th Century)
Four historical periods for materials science and engineering
Stone Age
2.6 million years ago – 3300 B.C. Began about 2 million years ago.
Stone
Wood
Clay
Skins
Bronze Age
3300 B.C. – 1200 B.C.
Began about 5000 years ago
An alloy which is made up of more than one element, copper + <25% of tin + other elements.
Can be hammered or cast into a variety of shapes, can be made harder by alloying, corrode only slowly after a surface oxide film forms.
Iron Age
1200 B.C – 600 B.C.
Began about 3000 years ago (until today)
Use of iron & steel, a stronger and cheaper material changed drastically daily life of a common person)
Advanced Materials (early 20th Century)
Throughout the Iron age many new types of materials have been introduced (ceramics, semiconductors, polymers, composites...)
Understanding of the relationship among structure, properties, processing and performance of materials.
Intelligent design of new materials
Materials Science
Investigates relationships that exist between the structures, processing and properties of materials.
Develop or synthesize new materials
Material Chemistry
Materials Physics
Materials and Process Engineering
Scope of Material Science
Graphite
A material that is dull, opaque, soft, and common.
Diamond
A material that is brilliant, transparent, hard, and rare.
Materials Engineering
Design the structure of a material to produce a predetermined set of properties.
Materials Engineering
Create new products or systems using existing materials
Develop techniques for processing materials.
Materials Engineering
Focuses on how to translate or transform materials into useful devices or structures.
Material Science
Basic knowledge of materials
Material Science and Engineering
Resultant knowledge of the structure, properties, processing and performance of engineering materials.
Materials Engineering
Applied knowledge of materials
Subatomic level
Electronic structure of individual atoms that defines interaction among atoms (interatomic bonding).
Atomic Level
Arrangement of atoms in materials (for the same atoms can have different properties, e.g. two forms of carbon: graphite and diamond)
Microscopic Structure
Arrangement of small grains of material that can be identified by microscope.
Macroscopic Structure
Structural elements that may be viewed with the naked eye.
Physical Properties
Properties that can be observed or measured WITHOUT changing the composition of the material.
Density
Thermal
Electrical
Dimensional
Optical • Refractive index • Absorption • Transmission • Reflection • Scattering • Color
Magnetism
Permeability
Porosity
Mechanical Properties
Properties that involve a reaction to an applied load.
Response to mechanical forces, strength, etc
Hardness
Toughness
Elasticity
Plasticity
Ductility
Malleability
Brittleness
Tensile strength
Compressive strength
Shear strength
Fatigue strength
Impact resistance
Creep
Stiffness
Resilience
Chemical Properties
Properties that are discovered by observing chemical reactions.
Reactivity with acids
Reactivity with bases
Oxidation state
Corrosion resistance
Flammability
Toxicity
pH level
Heat of combustion
Electronegativity
Chemical stability
Radioactivity
Ability to tarnish
Enthalpy of formation
Solubility
Decomposition
Resistivity
A measure of the resistance of a material to electrical conduction.
Thermal Properties
Properties of a material that is related to its ability to conduct heat.
Thermal conductivity
Specific heat capacity
Thermal expansion
Melting point
Boiling point
Thermal diffusivity
Heat of fusion
Heat of vaporization
Thermal emissivity
Glass transition temperature
Thermal shock resistance
Electrical Properties
Properties of a material that is related to its ability to conduct electricity.
Electrical conductivity
Electrical resistivity
Dielectric constant
Dielectric strength
Permittivity
Electrical permeability
Power factor
Insulation resistance
Hall effect
Superconductivity
Extrinsic and Intrinsic Factors
Two Size Effect Factors
Processing
Purified Ore - Metal compound separated from bits of sand and rock
Extracted - Metal chemically separated from other elements
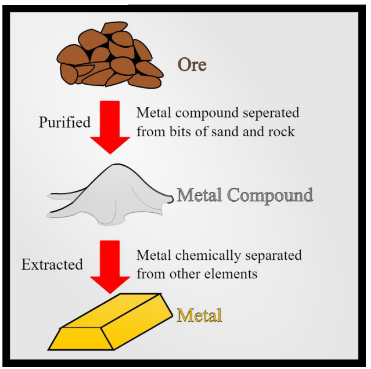
Extrinsic Factors
Geometry size
Surface morphology
Local feature size
Aspect ratio of parts
Intrinsic Factors
Grain size
Size/distance of precipitates
Material density
Dislocation mean free path
Interdependency between structure, properties, processing and performance of materials
Relationships in the material tetrahedron
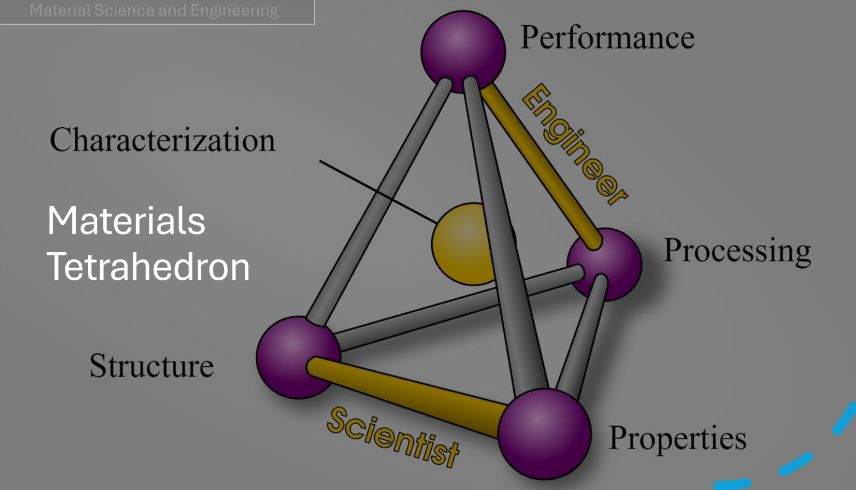
Structure
Arrangement of constituents or its internal components
Depends on how it is processed

Properties
Strength, ductility, toughness, stiffness, corrosion resistance, creep resistance, etc.
A materials trait in terms of response to a specific environment and external forces
Processing
Fabrication, accuracy, surface finish, cost, and required quality, etc
Way the materials are made; synthesized, produced and integrated
What are the parameters involved (source of energy, duration, stresses experienced etc)
Will indicate the properties
Performance
Intended service environment, reliability, expected service life, frequency of failure, remaining life assessment,etc
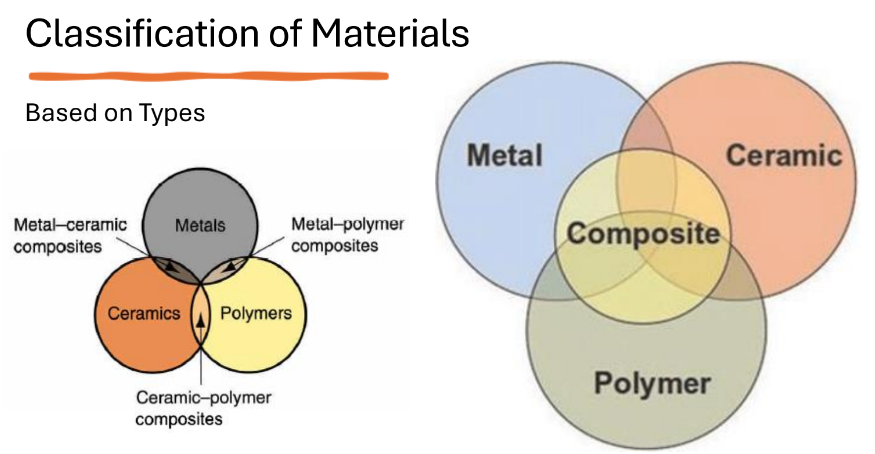
Metal and alloys
Ceramics
Composites
Polymers
Classification of materials based on TYPES
Metal and Alloys
Steel
Bronze
Brass Aluminum
Nichrome
Titanium
Berrylim-copper
Nickel
Niobium
Metals
Strong, ductile
High thermal & electrical conductivity
Opaque, reflective.
Examples: Beverage can: Aluminum alloy highly hardenable)
Deterioration
In the context of corrosion, is a loss in the properties of a material by chemical interaction with the environment
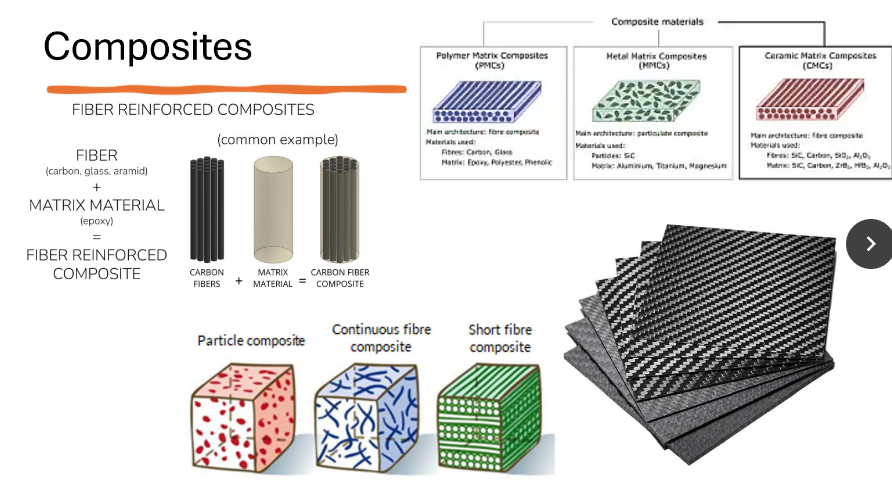
Composites
Metal Matrix
Ceramic Matrix
Polymer Matrix
Ceramics
Brittle, glassy, elastic
Non-conducting (insulators)
Crystaline Ceramics

Ceramics
An inorganic non-metallic solid made up of either metal or non-metal compounds (oxides, carbides, nitrides, sulfides) that have been shaped and then hardened by heating to high temperatures.
In general, they are hard, corrosion-resistant and brittle.
Composites
A combination of two or more materials (metals, ceramics and polymers) to form a new one.
To achieve a combination of properties that is not displayed by any single material.
Composites
One type of material will become the base; called ‘matrix’ and the other material will act as ‘reinforcement’.
Natural and Synthetic Polymers
Two types of Polymer
Polymers/plastics
Soft, ductile, low strength, low density
Thermal & electrical insulators
Optically translucent or transparent.
They decompose at moderate temperatures (100 – 400 C).
Polymers
A substance or material consisting of very large molecules, or macromolecules, composed of many repeating subunits. Intramolecularly bonded by covalent bonding.
Wires & cables
Ski boot
Modern telecommunications equipment
Natural Polymers
Polymers made by living organism.
Synthetic Polymers
Polymers made by chemical reaction in a laboratory.

Aerospace
Biomedical
Electronics
Magnetic
Optical
Structural
Smart Materials
Classification of materials based of FUNCTIONS
Functional materials
Generally characterized as those materials which possess particular native properties and functions of their own.
Aerospace
Engine, turbo fan (metals and alloys), wheels (synthetic polymers, aircraft (composites)
Biomedical
- implant, medicines
Electronics
- electronic board (ceramic matrix composites)
Magnetic
power magnetics (used in transformer), electromechanical component (hard drive)
Optical
optical lenses
Structural
bricks, cement, sand, concrete
Smart Materials
ferrofluid, metal foam, aerogel, nanotube
Stone
Bronze
Iron
Advanced Materials
Historical Perspective
Structure
Properties
Processing
Performance
Materials Science and Engineering
Earliest humans
Access to only limited number of materials those that naturally occur (stone, wood, clay, skins etc.)
Pottery and Metals
Earliest humans discovered techniques for producing materials that had properties superior to those of the natural ones. What is this?
Pottery
Made by forming a CLAY body into objects and heating them to high temperatures
Density
Implies the weight of a material, with higher __________ rates implying heavier materials.
Atomic Structure
Bohr Theory Model (orbital electron)
Wave-Mechanical Model (electron configuration)
Isotopes
Atoms of some elements have two or more different atomic masses
ATOMIC NUMBER (Z)
Number of protons is equal to
MASS NUMBER (A)
No. of protons + no. of neutrons (A = Z +N)
Atomic Mass Unit (AMU)
Unit of mass relative to a single constituent of a Carbon atom
Principal (n)
Size of the shell in which a particular electron orbits arounf the nucleus and its energy.
Secondary/ Azimuthal (l)
Specifies the angular momentum of orbiting electron and determine the shape of the orbit
Magnetic (mₗ)
Controls the number of allowed spatial orientations characterized by l in a given shell.
Pauli Exclusion Principle
No two electrons may have the same quantum number within any atom.
No more than two electrons with opposing electronic spin may be present in each orbital
Electropositive
Electronegative
Electronegativity
giving up electrons to become + ions
accept electrons to form - ions
increases in moving from left to right and from bottom to top
Ionic bond
Transfer of electron between metals and non-metals
Nondirectional, magnitude of the bond is equal in all directions around an ion
Electrically and thermally insulative
High Melting Point, Brittle, Hard
Covalent bond
Sharing an electron between two similar electronegative elements
Electrical insulators or semiconductor
High Melting Point, Hard, Nonconducting
Metallic bonding
Formed between the metallic elements in which electropositive atoms donate their valence electrons to form a “sea” of electrons
Good conductors of electricity and heat
Intermolecular Forces
Attraction forced between molecules
Dipole
Polarized molecule having partially positive and negative poles.
Amorphous
Does not have a long-range order
Single crystals
One crystal
Polycrystalline
Many crystals or grains
Grain boundaries
Regions between individual crystals
Magnetic materials
Those materials that can be easily magnetized and can be used to make magnets.
Examples are iron, nickel and steel.
Diamagnetic Materials
Paramagnetic Materials
Ferromagnetic Materials
3 Primary Categories Of Magnetic Materials
Diamagnetic Materials
Materials which are weakly repelled by a magnet;
Magnetized in opposite direction;
when freely suspended in a uniform magnetic field it slowly aligns itself in a direction perpendicular to the applied magnetic field;
lose their magnetism on removal of external magnetic field;
in a non-uniform magnetic field
they move from a stronger to a weaker field due to repulsion
examples: copper, gold, silver, water, air, argon, hydrogen.
Paramagnetic Materials
materials which are weakly attracted by a magnet;
weakly magnetized in the same direction of magnetic field;
when freely suspended in a uniform magnetic field it slowly aligns itself parallel to the applied magnetic field;
also lose their magnetism on removal of external magnetic field;
move from a weaker to a stronger field with a weaker attraction;
examples: aluminium, chromium, alkaly, metals, alkaline, earth metals, platinum, oxygen
Ferromagnetic Material
materials which are strongly attracted by a magnet;
strongly magnetized in the same direction;
when freely suspended in a uniform magnetic field it quickly aligns itself parallel to the applied magnetic field;
DO NOT lose their magnetism on removal of external magnetic field (THEY ARE A PERMANENT MAGNET);
move from a weaker to a stronger field due to strong attraction;
examples: iron, cobalt, nickel, steel