MAIN Anemia Flashcards (Dr. Kamuyango)
1/84
Earn XP
Description and Tags
Combined with megaloblastic flashcards
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
What is haemopoiesis
Haemopoiesis is the process by which blood cells are produced, including red blood cells, white blood cells, and platelets, primarily in the bone marrow.
What is the pneumonic to remember the types of WBCs
"Never Let Monkeys Eat Bananas"
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
What is the meaning of intra/extramedullary
Intra/extramedullary refers to the locations within or outside the bone marrow where haemopoiesis occurs. Intra means within the marrow, while extra signifies outside the marrow.
What are the reasons we need haemopoiesis
maintaining adequate levels of blood cells
facilitating oxygen transport,
immune defence
clotting.
It replaces aged or damaged cells
What is the meaning of a haemoproliferative disorder and an example
A haemoproliferative disorder is a condition where there is an abnormal increase in the number of blood cells, typically in the bone marrow. An example includes leukaemias
Where are the sites of foetal haemopoiesis and the age ranges of when it happens
0-2 months (yolk sac, aka vitelline sac)
2-7 months (liver, spleen)
5-9 months (bone marrow)
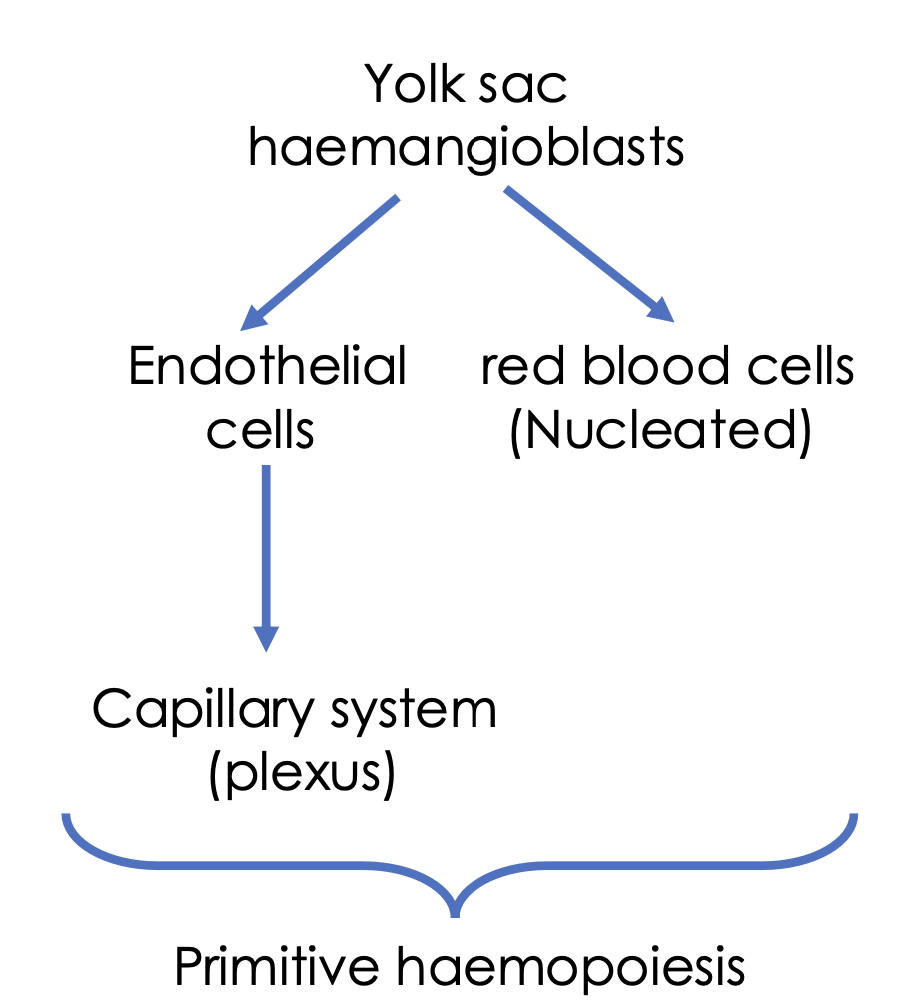
Where are the sites of infant haemopoiesis
Bone marrow (practically all bones)
Where are the sites of adult haemopoiesis
Predominately in bone marrow (Primarily the sternum, femurs and pelvis)
Occasionally – lymph nodes, liver and spleen
What are the three components of the bone marrow architecture
Haemopoietic tissue (cords)
Stem cells and the immature cells
Non-haemopoietic cells
Fibroblasts (collagen – to support other cells)
Macrophages – produce growth factors, store iron
Adipocytes – fat laden to store energy
Sinuses
Vascular spaces lined with endothelial cells
Regulate the release of cells into the blood
What is an example of haemopoietic tissue
Bone marrow or lymphoid tissue. These tissues are critical for the production and maturation of blood cells.
Explain what are sinuses
Vascular spaces within bone marrow lined with endothelial cells that regulate the release of blood cells.
Explain properties of stem cells
Self-renewal
Proliferate and differentiate into different blood cell types via lineage-specific progenitor cells
1 in every 20 million nucleated cells in the marrow is a pluripotent stem cell
What are the specific markers for stem cells and why are they important
Specific markers
CD34+
CD59+,
CD38-
Important for stem cell transplantation
What are colony-forming units (CFUs)
Colony-forming units (CFUs) are progenitor cells that give rise to various types of blood cells in the bone marrow, capable of forming colonies in culture.
What are the 3 types of colony-forming units (CFUs)
CFU-GEMM (Granulocyte, Erythrocyte, Monocyte, Megakaryocyte)
CFU-L (Lymphoid)
BFU-E (Burst Forming Unit-Erythroid)
What are blast cells, where are they found and examples
Blast cells are immature precursor cells found predominantly in the bone marrow that give rise to various types of blood cells
In adults – exclusively found in the bone marrow
Seen in peripheral blood in certain diseases i.e. leukaemia
Myeloblast; lymphoblast, rubri/erythroblast
Have the greatest mitotic activity
What 2 things regulates haemopoiesis
Bone marrow microenvironment
Position of cells in the bone marrow
Cytokine growth factors
Proteins and glycoproteins
Regulate proliferation and differentiation of progenitor cells
What growth factors act on:
a. Stromal cells
b. PSCs
c. Multipotential progenitor cells
d. Committed progenitor cells
Stromal cells. IL-1, TNF
PSCs. SCF, FLT3-L, VEGF
MPCs. IL-3, GM-CSF, IL-6, G-CSF, Thrombopoietin
CPC. G-CSF, M-CSF, IL-5, Erythropoietin, Thrombopoietin,
What is the meaning of erythropoiesis
Erythropoiesis is the process of producing red blood cells (erythrocytes) from progenitor cells in the bone marrow, regulated by erythropoietin and influenced by oxygen levels.
What is the erythropoiesis cycle of production
Erythropoiesis cycle refers to the series of stages through which progenitor cells in the bone marrow mature into erythrocytes.
This includes differentiation, maturation, and release into the bloodstream, influenced by erythropoietin and oxygen levels.
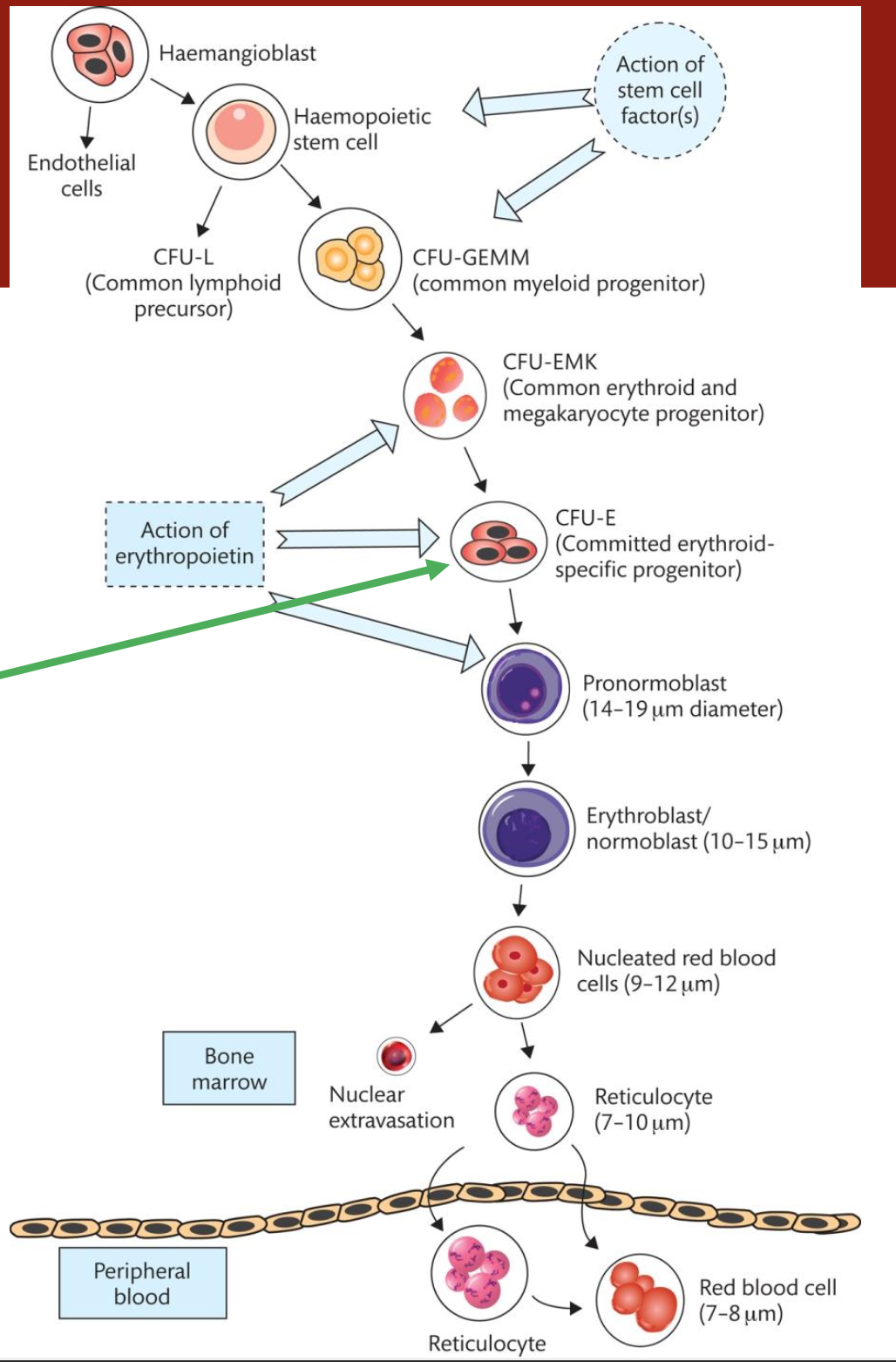
What are the meanings of these terms:
Poikilocytosis
Anisocytosis
Polychromasia
Spherocytes
Poikilocytosis - variation in red blood cell shapes
Anisocytosis - size variation of red blood cells
Polychromasia - presence of varying colours in red blood cells
Spherocytes - presence of spherical red blood cells
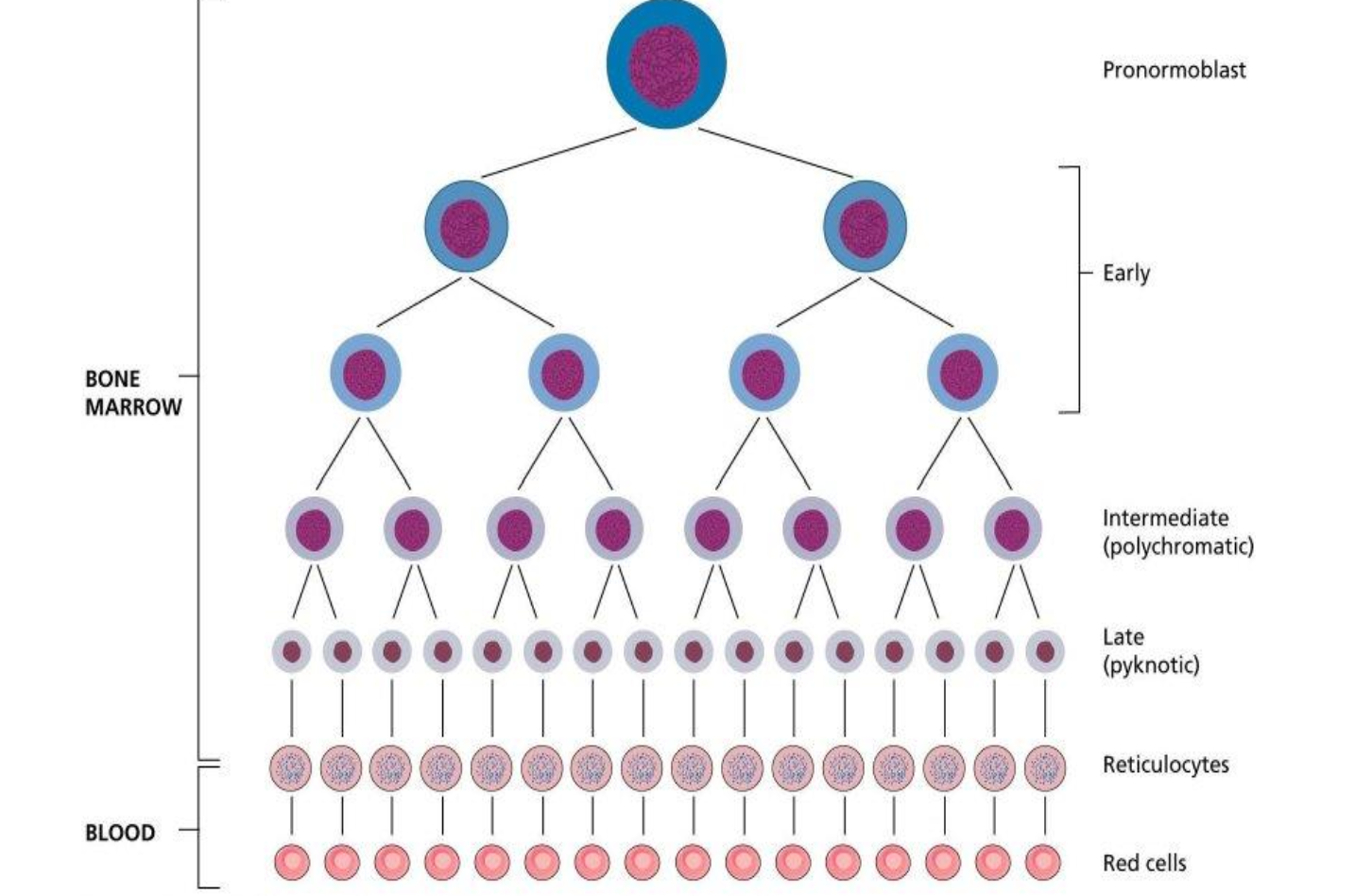
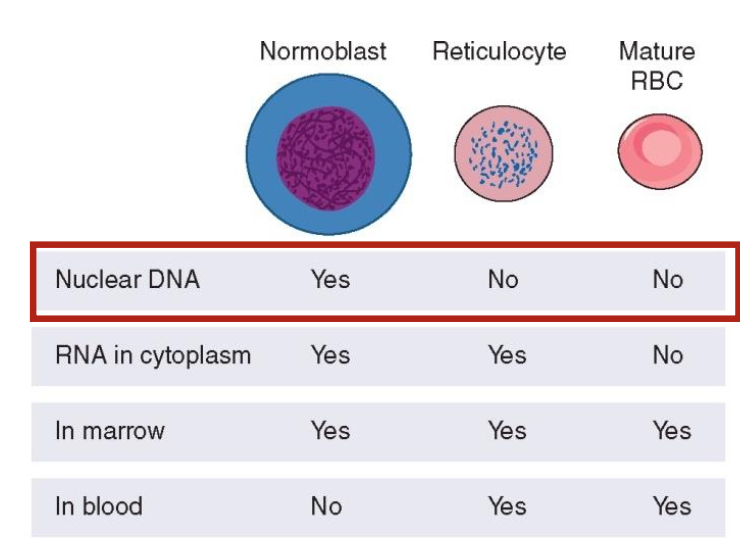
What is the comparison of the DNA and RNA content between normoblast, reticulocytes and mature RBCs
Normoblasts contain abundant DNA
RNA, reticulocytes have diminished RNA as they lose organelles
Mature RBCs primarily lack DNA and have minimal RNA.
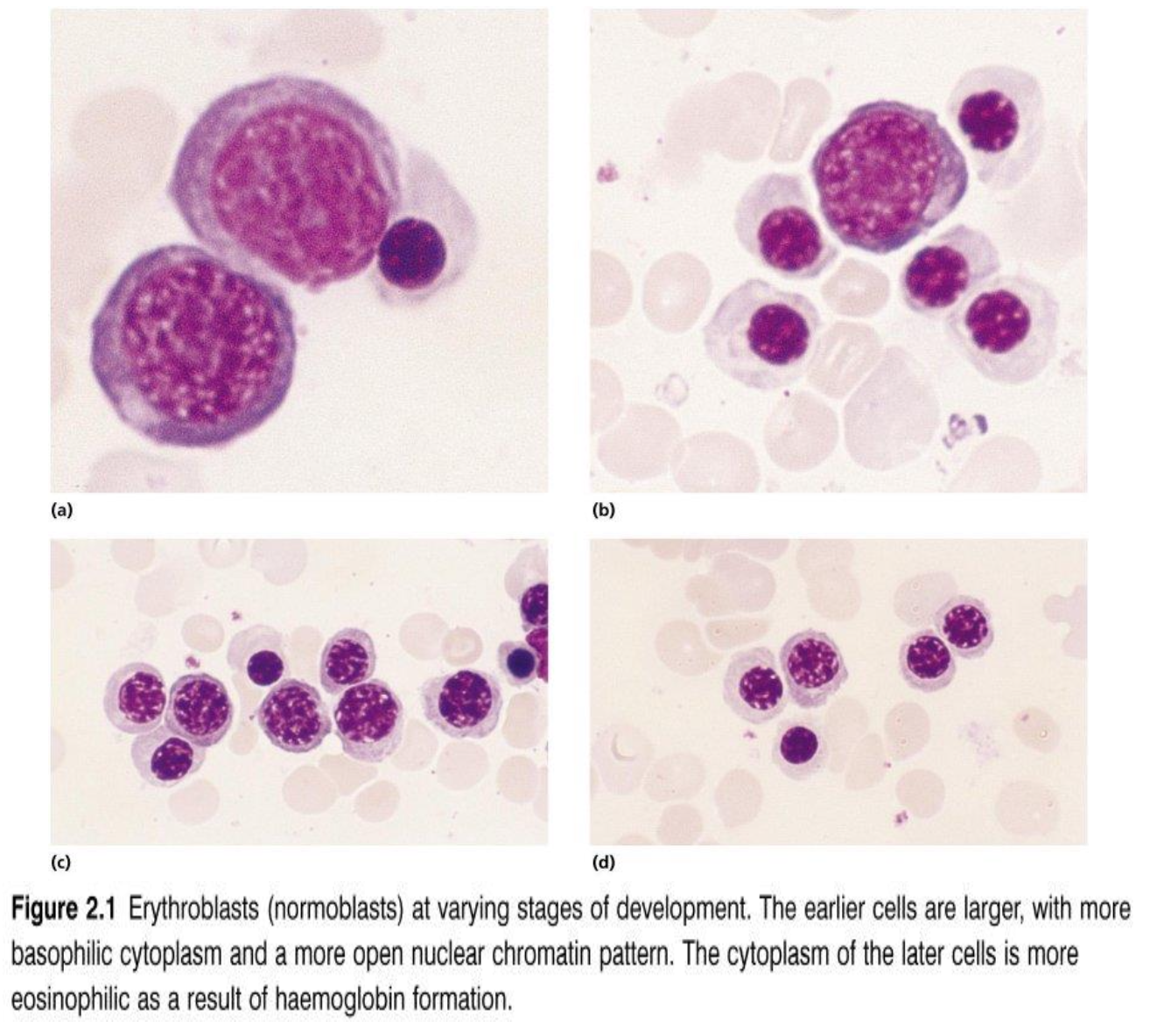
What are the characteristics of precursor cells
The earlier cells are larger with more basophilic cytoplasm and a more open nuclear chromatin pattern.
The cytoplasm of the latter cells is more eosinophilic as a result of haemoglobin formation
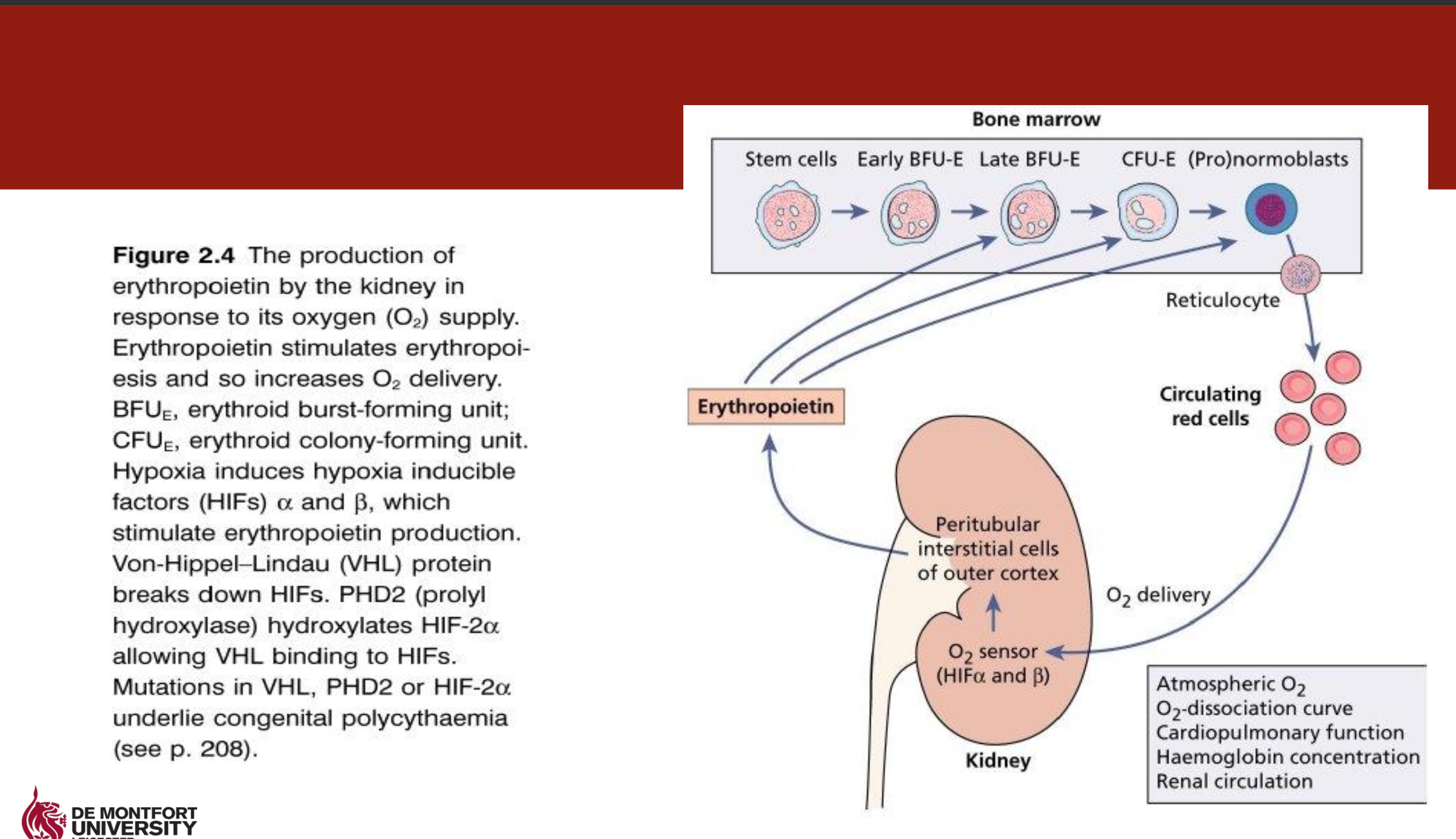
What is Erythropoietin (EPO)
Acts on pronormoblast, early normoblast, polychromatic normoblasts, reticulocytes, & erythrocytes
Production 90:10 kidneys:liver
Heavily glycosylated 34kDa protein
EPO production is stimulated by tissue hypoxia via hypoxia-inducible factors (HIF-α and β)
Haemorrhage or anaemia results in increased levels of EPO
Produced EPO binds to immature erythroid cells leading to increased replication and maturation of RBCs.
Clinical relevance
EPO usually low in individuals with renal disease
Low EPO lead to anaemia
How is RBCs cleared form the body
Removed by liver and spleen after 120 days
As the erythrocyte ages surface proteins particularly “band 3” are progressively oxidised
This provides a target for phagocytosis by macrophages lining liver & spleen sinuses.
What are Nucleated RBCs and its clinical conditions and formation
Nucleated red blood cells (nRBCs) — also called erythroblasts — are immature red blood cells that still contain a nucleus.
Normally, these cells mature in the bone marrow, expelling their nuclei before entering the bloodstream.
Their presence in peripheral blood is abnormal after the neonatal period and usually indicates stress on the bone marrow or severe systemic conditions.
What are Howell Jolly bodies and its clinical conditions and formation
Howell-Jolly bodies are small, round basophilic nuclear remnants (DNA fragments) found inside red blood cells.
They are normally removed by the spleen, so their presence in peripheral blood is a key indicator of splenic dysfunction or absence.
What is basophillic stripping and its clinical conditions and formation
Basophilic stippling refers to the presence of small, dark blue granules scattered throughout the cytoplasm of red blood cells when stained with a Romanowsky-type stain (like Wright or Giemsa).
These granules represent aggregated ribosomal RNA and are not normally seen in mature RBCs.
What are Pappenhemier bodies and its clinical conditions and formation
Pappenheimer bodies are iron-containing granules seen in red blood cells on a peripheral blood smear.
They are abnormal aggregates of ferritin or hemosiderin and are found in RBCs when iron metabolism is disturbed or there is ineffective erythropoiesis.
What is heinz bodies and its clinical conditions and formation
Heinz bodies are inclusions of denatured or precipitated hemoglobin found inside red blood cells.
They form when hemoglobin is damaged by oxidative stress, leading to the precipitation of globin chains within the RBC.
What is reticulocyte
A reticulocyte is an immature red blood cell that contains a network of ribonucleic acid (RNA). It is a crucial indicator of bone marrow activity and is increased in conditions like hemolytic anemia or after blood loss.
What is anaemia
A condition characterised by a deficiency of red blood cells or haemoglobin in the blood, leading to reduced oxygen transport to tissues.
What are some of the general symptoms of anemia
Decreased work capacity, fatigue, lethargy
Weakness, dizziness, palpitations
Shortness of breath (especially on exertion)
‘Tired all the time’
In children: decreased IQ, poor concentration and sleepiness
Rarely: Headache, tinnitus, taste disturbance
More severe diseases
Jaundice, splenomegaly
Hepatomegaly, angina, cardiac failure, fever
What are some of the general signs of anemia
Pallor (especially the conjunctiva)
Tachycardia (pulse rate over 100 beats per minute)
Glossitis (swollen and painful tongue – reasonably specific for vitamin B12 deficiency)
Koilonychia (spoon nails – reasonably specific for iron deficiency anaemia)
Dark urine (in haemolytic anaemia)
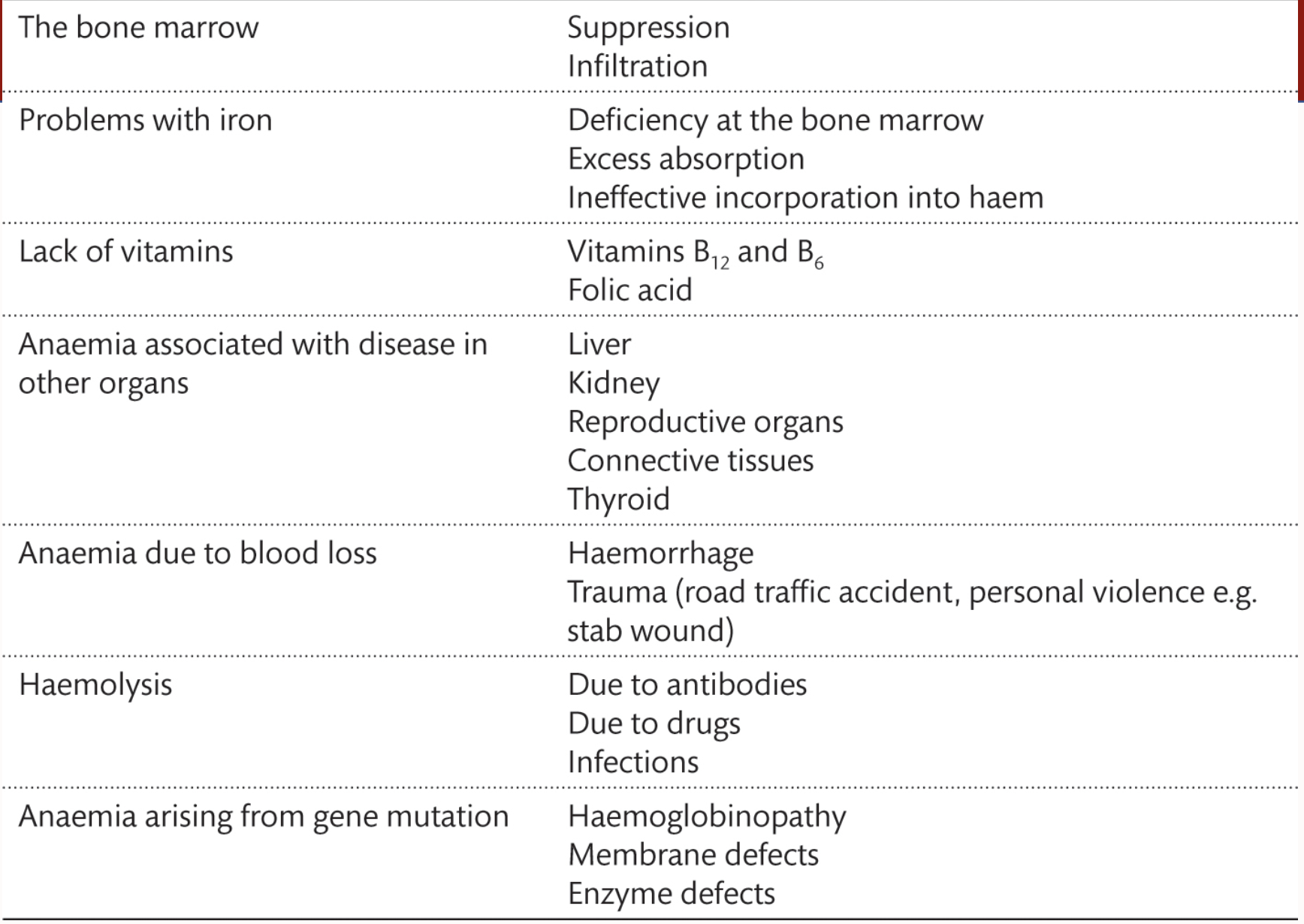
What is the aetiology of anaemia
Lack of vitamins
Suprression/Inflitration in the bone marrow
Gene muations
more on the picture
What are the meanings of these words:
MCV
MCH
Mean CELL Volume (MCV) is a measure of the average volume of red blood cells
Mean CELL Hemoglobin (MCH) indicates the average amount of haemoglobin per red blood cell.
What are the three classifications of anaemia according to MCV
Microcytic
Normocytic
Macrocytic
What types of specific anaemias relate to each of the classifications
Microcytic
Normocytic
Macrocytic
Microcytic anaemia includes iron deficiency anaemia and thalassemia.
Normocytic anaemia includes aplastic anaemia and anaemia of chronic disease.
Macrocytic anaemia is primarily associated with vitamin B12 deficiency and folate deficiency.
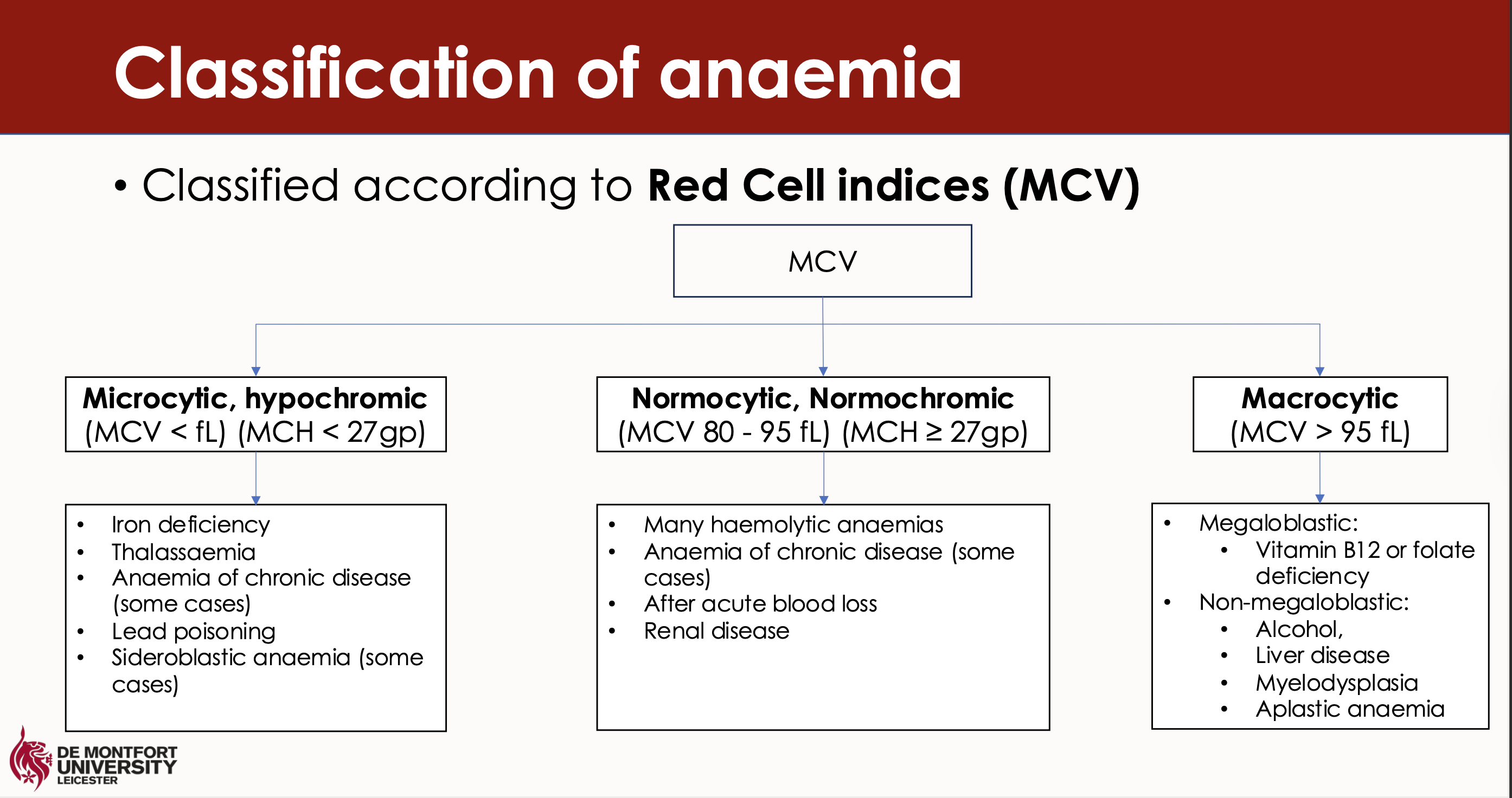
What are some of the lab tests to detect anaemia
Red cell indices
Leucocyte and platelet count – distinguish pure anaemia from pancytopenia
Reticulocyte count – usually increased in anaemia due to erythropoietic increase
Blood film – look for abnormal red cell morphology or red cell inclusion bodies
Bone marrow – only needed when cause cannot be identified using other tests
What is iron deficiency anemia
A condition characterised by insufficient iron levels, leading to decreased haemoglobin production, resulting in microcytic and hypochromic red blood cells.
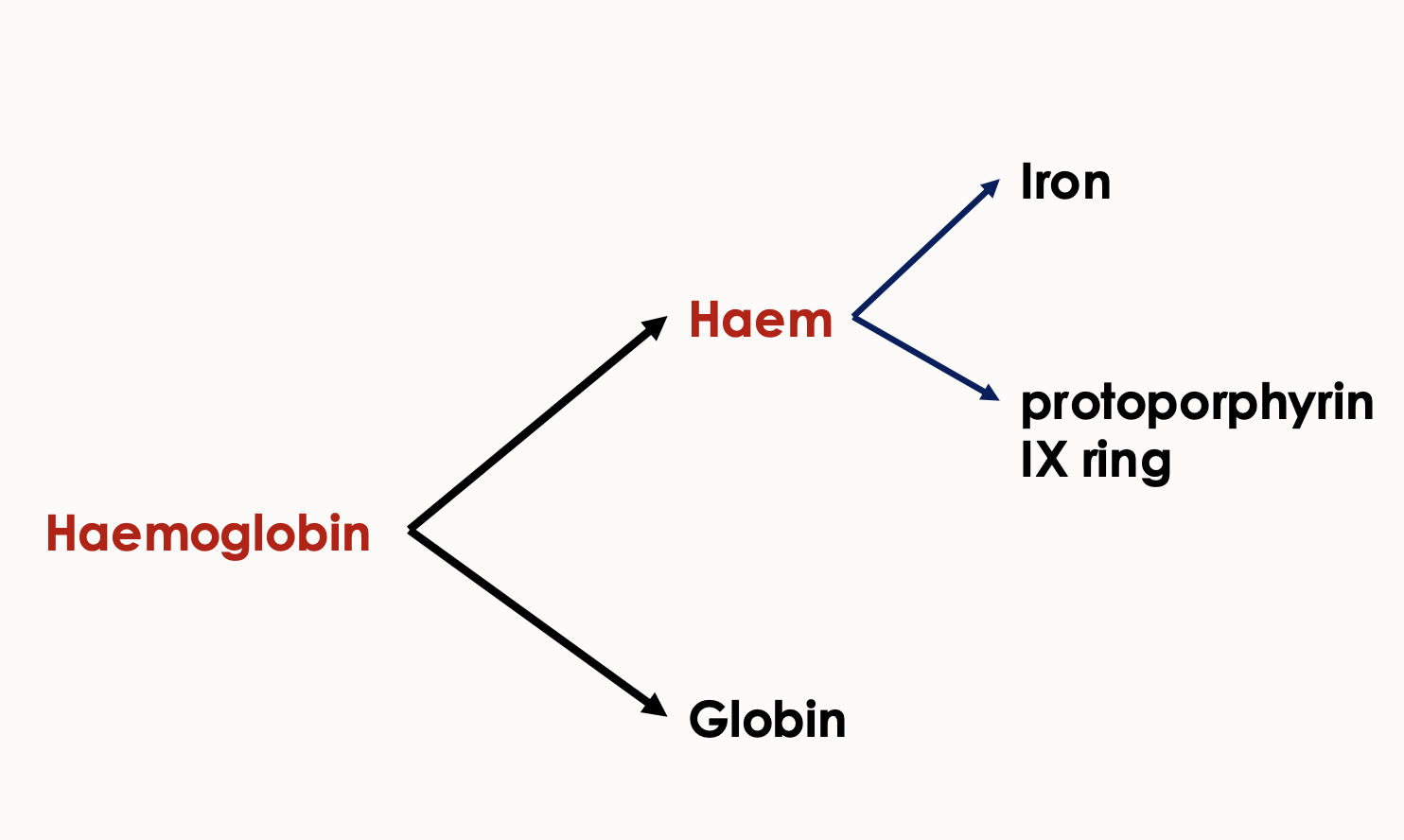
What are the two components that make:
Haemoglobin
Haem
haemoglobin = haem + globin
haem = iron + protoporphyrin IX ring
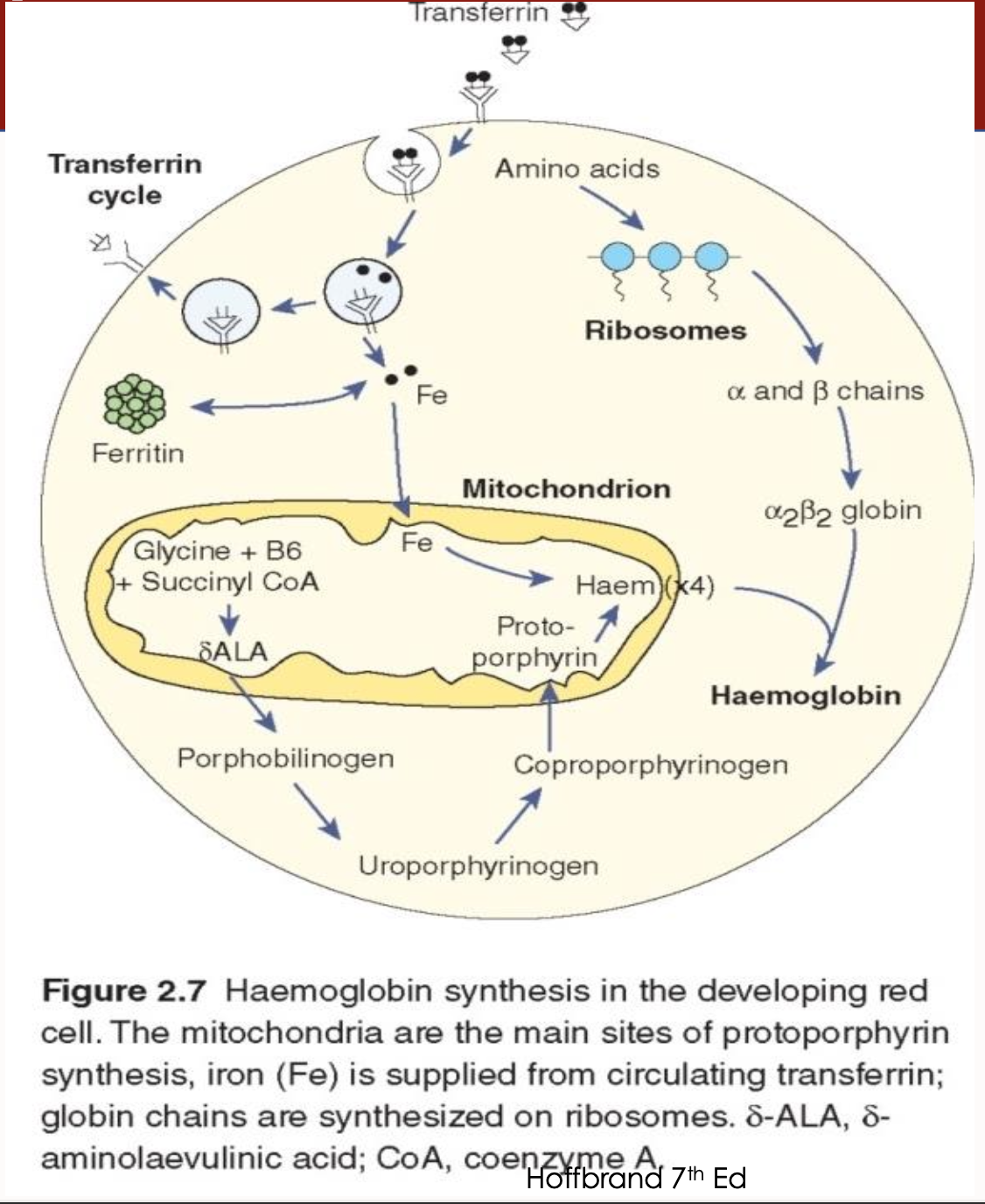
What is the cycle for haemoglobin synthesis in erythroblast
Transferrin binds iron (2 atoms)
Transferrin receptors are present on the erythroblast surface for iron uptake
Transferrin docks to the transferrin receptor
Transferrin is then recycled
What is non-haem stored as and its location
Non-heme iron is stored in the liver and marrow as ferritin and haemosiderin
What is the aetiology of iron deficiency anaemia
Iron-deficient diet
Inadequate intestinal iron absorption
Chronic blood loss (menorrhagia or gastrointestinal bleeding)
Liver disease- failure to produce adequate transferrin)
Intravascular haemolysis
What are some factors Favouring affecting iron absorbtion
Vitamin C (Ascorbic acid) | Converts ferric (Fe³⁺) to ferrous (Fe²⁺) form, which is more absorbable. |
Gastric acid (HCl) | Low pH helps solubilize iron and keeps it in the ferrous form. |
Meat, Fish, Poultry (MFP factor) | Contains heme iron (more easily absorbed) and enhances non-heme iron absorption. |
Lactoferrin (in breast milk) | Binds iron and facilitates absorption, especially in infants. |
Fermented foods | Reduce phytate content and improve bioavailability. |
Low body iron stores | Increases expression of iron transporters in the gut. |
What are some factors affecting/reducing iron absorption
Phytates (e.g. in grains, legumes) | Bind iron and form insoluble complexes. |
Polyphenols (e.g. in tea, coffee, red wine) | Strongly chelate non-heme iron, reducing absorption. |
Calcium (e.g. in dairy) | Competes with iron for absorption (especially non-heme). |
Oxalates (e.g. in spinach, rhubarb) | Form insoluble iron complexes. |
Excess zinc or manganese | Compete for the same transporter as non-heme iron. |
Antacids/PPIs | Reduce stomach acid, decreasing solubility of iron. |
Inflammation or infection (↑ hepcidin) | Hepcidin reduces iron release and absorption during illness. |
What are the 3 stages of iron deficiency stages
Iron depletion
Iron-deficient erythropoiesis
Iron deficiency anaemia
What are the clinical features related to iron deficiency anaemia
Koilonychia
Angular cheilitis
Pica
Children - irritability, reduced psychomotor development
What are the lab findings in these tests for iron deficiency anaemia
a. FBC
b. RBC morphology
c. IDA biochem test
Low MCV and MCH
Microcytic hypochromic red blood cells
Low serum ferritin and low serum iron with high total iron binding capacity (TIBC)
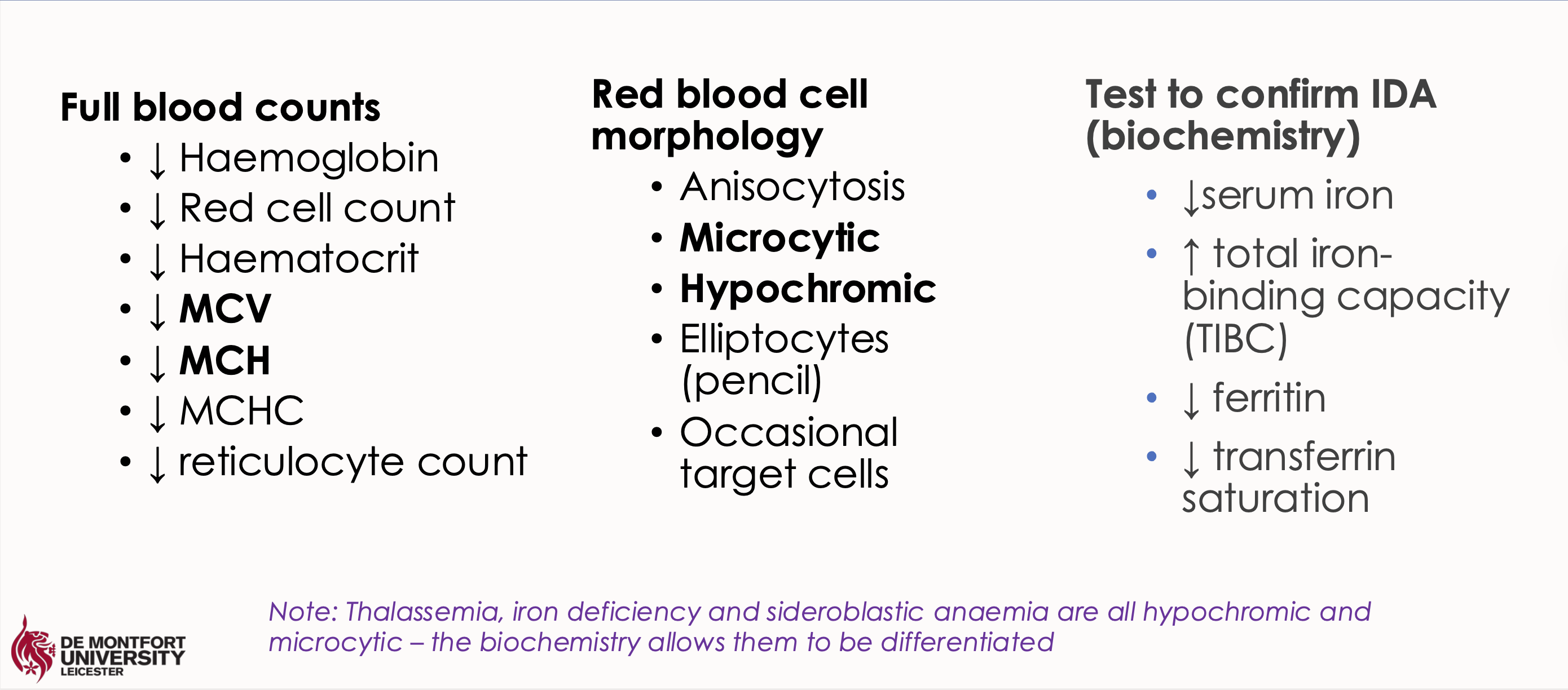
What happens in hypochromasia in iron deficiency anaemia
Low HGB and MCH - hypochromasia
Fe is needed for the production of haemoglobin
Less haemoglobin leads to hypochromic cells (paler than normal)
Less HGB means fewer red cells are made means low RBC
What happens in microcytosis in iron deficiency anaemia
Low MCV - microcytosis
The number of cell divisions during erythropoiesis is determined by the level of erythroblast haemoglobinization
Lack of haemoglobin leads to more mitotic divisions (an effort to maintain mean corpuscular Hb concentration)
This leads to smaller cells – microcytosis
And fewer cells – low RBC
What is the treatment against Iron deficiency
Oral iron – ferrous sulphate
67mg of iron / 200mg anhydrous
tablet
Side effects – nausea, abdominal
pain
What is the difference between:
Preliminary diagnosis
Differential diagnosis
Preliminary: Most likely diagnosis given the symptoms and initial lab findings while awaiting special or more definitive investigations.
Differential: List of other possible conditions or diseases that could be causing common symptoms or lab findings.
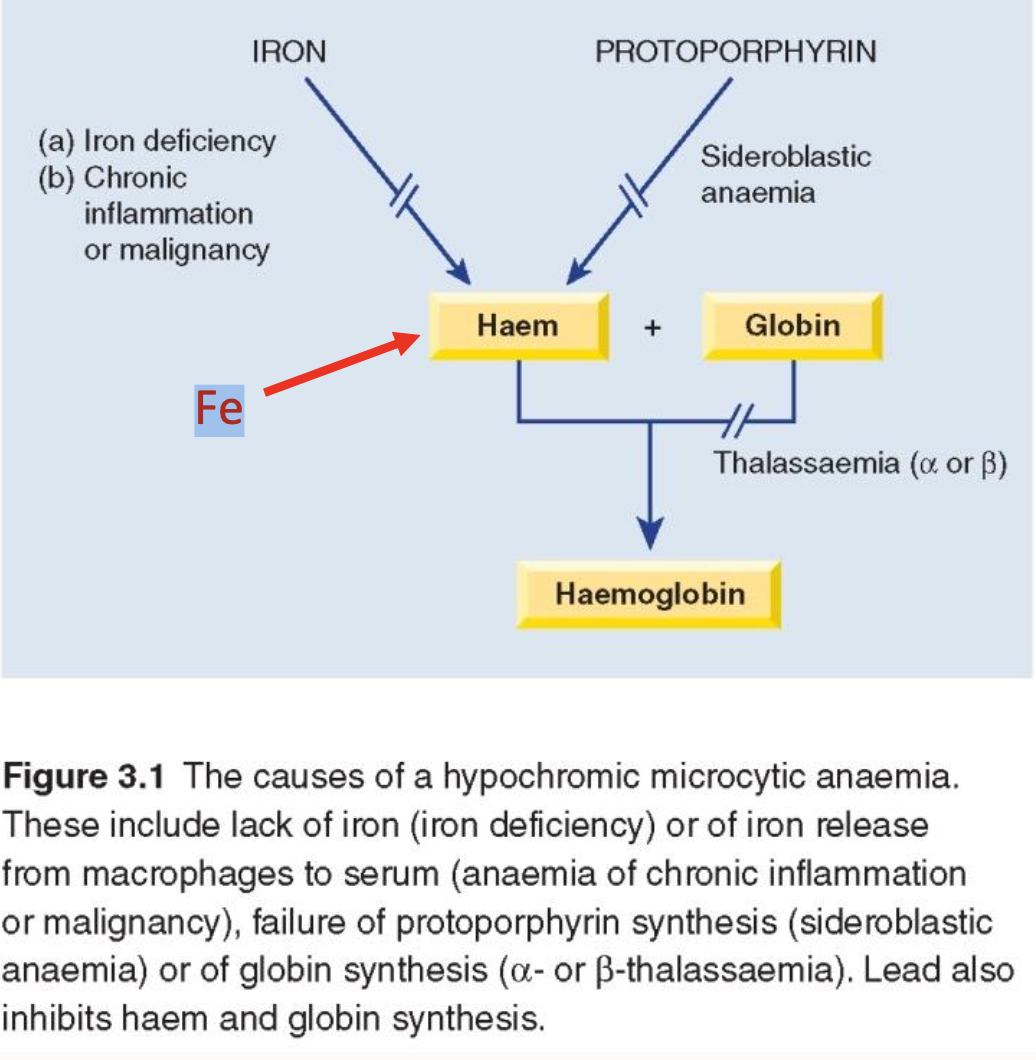
What are the differential diagnoses of hypochromic, microcytic anaemia
Iron deficiency
Thalassaemia
Anaemia of chronic disease (some cases)
Lead poisoning
Sideroblastic anaemia (some cases)
What are the lab tests for differentiating hypochromic, microcytic anaemias
These typically include a Complete Blood Count (CBC):
MCV/MCH
serum ferritin
serum iron,
total iron-binding capacity (TIBC),
haemoglobin electrophoresis
bone marrow iron stores
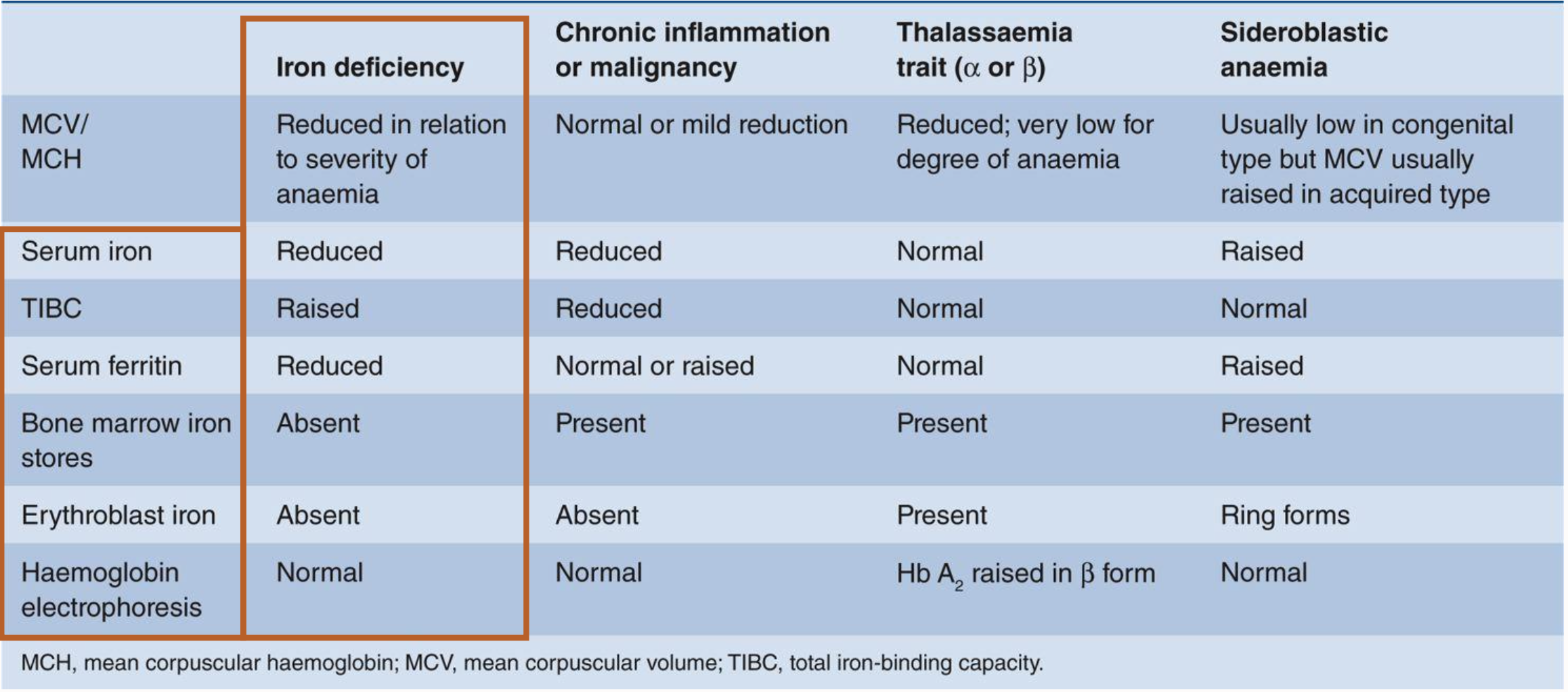
Megaloblastic Anaemia
A group of anaemias characterized by delayed maturation of the nucleus relative to the cytoplasm due to defects in DNA synthesis caused by dietary deficiency of folic acid or vitamin B12.
Hypersegmented Neutrophils
Neutrophils with more than the normal number of nuclei segments, often seen in megaloblastic anemia.
Vitamin B12
Also known as cobalamin, a vitamin crucial for DNA synthesis and neurological function, acquired primarily from animal products.
What is the in-depth biochemical function of Vit B12
Methyl B12 - acts as a cofactor of Methionine synthase (an enzyme responsible for methylation of homocysteine to methionine using methyl THF as a methyl donor
Deoxyadenosyl B12 (Ado B12) -acts as a cofactor of Methylmalonic mutase in conversion of Methylmalonyl CoA to Succinyl CoA
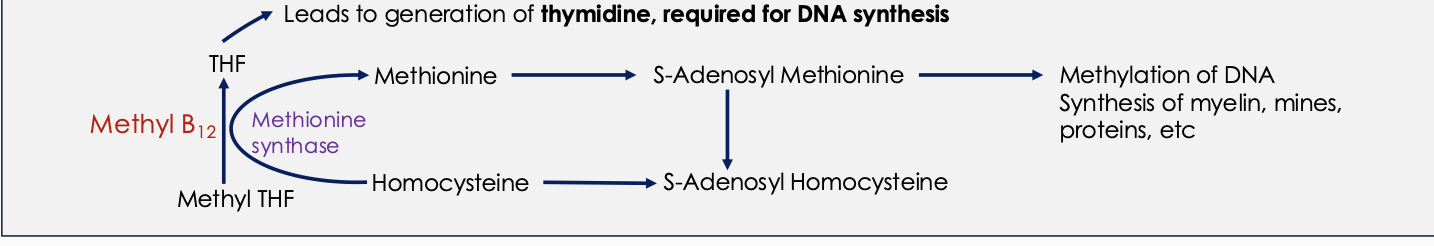
What is Vit B12 composed of
Ring of 4 pyrrole units
An atom of cobalt at its centre
Cobalamin
Another name for Vitamin B12, which is necessary for the metabolism of every cell of the human body, particularly affecting DNA synthesis.
Folate
Also known as pteroylglutamic acid, it is a B vitamin important for DNA and RNA production and cell division, obtained from dietary sources like fruits and vegetables. eggs and dairy,
What is the first stage of Vit B12 absorption process
Extracted from food by proteolytic enzyme pepsin and acid stomach environment
What is the second stage of Vit B12 absorption process
First vit b12 binds to haptocorrin (HC)
In the duodenum, B12 is released from HC by proteolytic action of pancreatic trypsin
What is the third stage of Vit B12 absorption process
And then binds to intrinsic factor (IF) (synthesized by gastric parietal cells) in the duodenum
What is the fourth stage of Vit B12 absorption process
IF-B12 complex is then absorbed in the distal ileum where the complex binds to the IF receptor (cubulin)
What is the final stages of Vit B12 absorption process
In blood Vitamin B12 binds to transcobalamin II (TCII)
• TCII takes B12 to the bone marrow and tissues
• Stored in the liver in sufficient amounts to last 6-12 months
Intrinsic Factor (IF)
A protein secreted by gastric parietal cells that is essential for the absorption of vitamin B12 in the intestine.
Macrocytic Anemia
A type of anemia characterized by the presence of abnormally large red blood cells, typically seen in Vitamin B12 and folate deficiency.
Peripheral Neuropathy
A condition resulting from damage to the peripheral nerves, commonly associated with severe Vitamin B12 deficiency.
Methylcobalamin
One of the active forms of Vitamin B12 found in the human plasma.
Deoxyadenosylcobalamin
One of the active forms of Vitamin B12 found in the human tissue.
Hydroxocobalamin
One of the active forms of Vitamin B12 used in treatment
Anisocytosis
A condition of having red blood cells of unequal size, which is often observed in various forms of anemia.
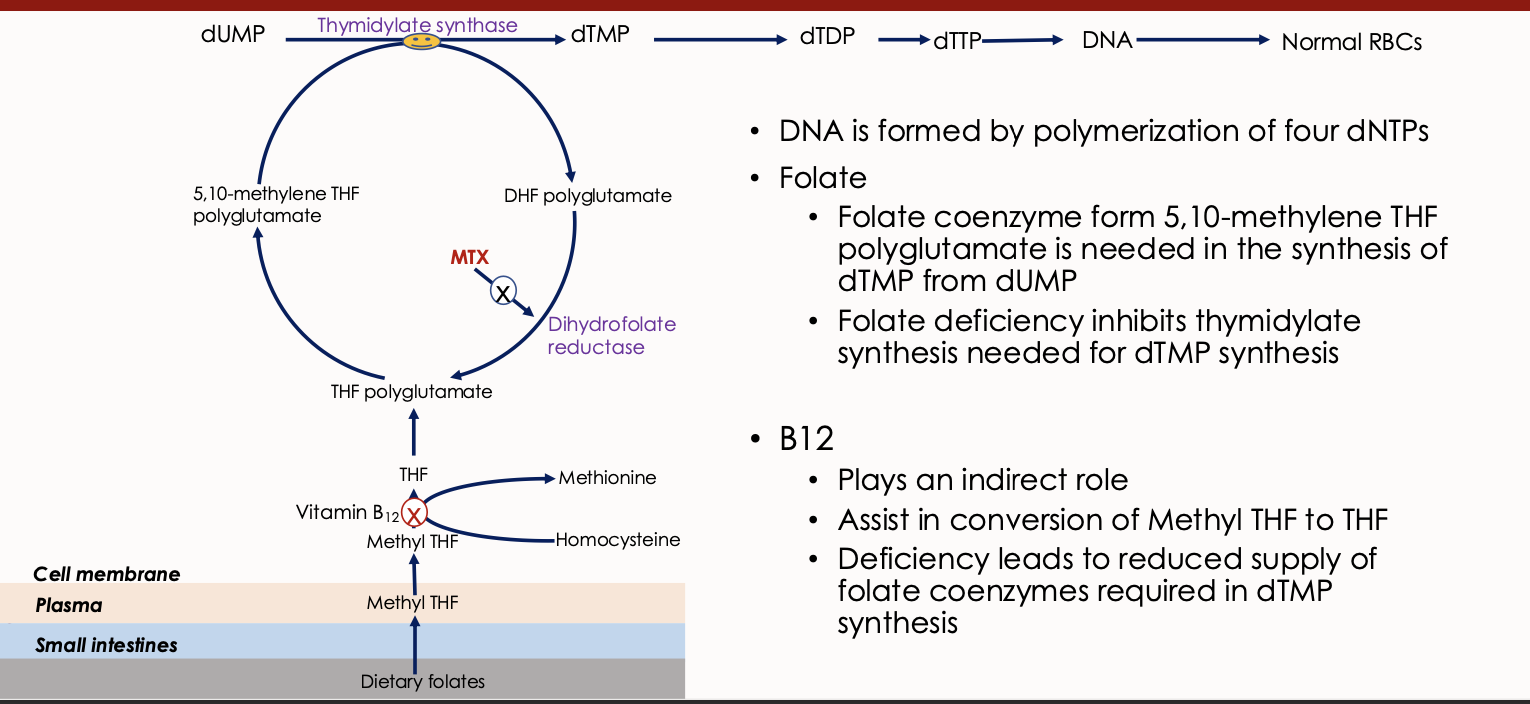
Thymidylate Synthase
An enzyme involved in DNA synthesis that converts dUMP to dTMP, requiring folate as a cofactor.
Pernicious Anemia
An autoimmune condition that causes a deficiency of intrinsic factor leading to Vitamin B12 malabsorption.
Dihydrofolate Reductase
An enzyme involved in the reduction of dihydrofolate to tetrahydrofolate, essential for DNA synthesis.
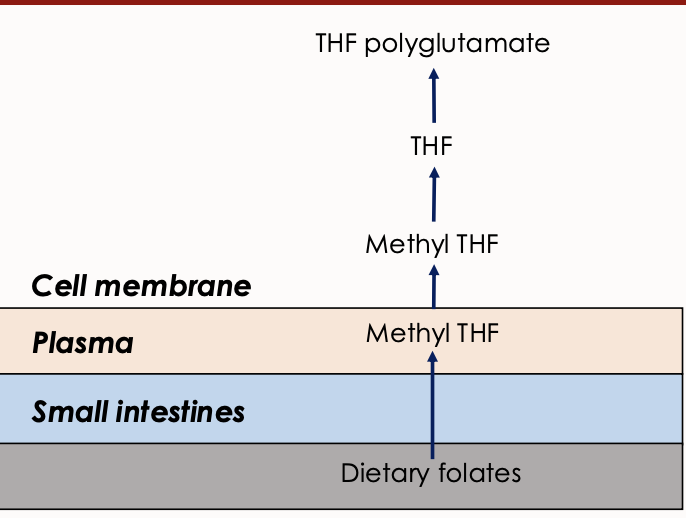
Dietary Folates
Forms of folate obtained from food, which undergo conversion to active forms during absorption. Dietary folates are converted to methyl tetrahydrofolate (THF) during absorption primarily through the duodenum and jejunum
In cell converted to folate polyglutamate
Megaloblasts
Abnormally large erythroblasts which are indicative of megaloblastic anemia.
Bone Marrow Examination
A diagnostic procedure used to investigate blood disorders, including the presence of megaloblasts in megaloblastic anemia.
Clinical features of Vit B12 deficiency
Jaundice
Glositis
Peripheral neuropathy
Fatigue
Spinal bifida
Angular cheilosis
What are the lab features of Vit B12 in full blood count
Reduced haemoglobin
increased MCV
the possibility of increased MCH and MCHC
What are the causes of B12 deficiency
Nutritional – strict veganism
Malabsorption: Gastric causes: pernicious anaemia, gastrectomy, congenital lack/abnormality of IF, use of proton pump inhibitors
Intestinal causes: Tropical sprue, fish tapeworm, ileal disease, bacterial overgrowth,
Pancreatic insufficiency
what are the causes of folate deficiency
Nutritional – especially old age, poverty etc
Malabsorption – tropical sprue, gluten induced enteropathy
Increased requirements caused by cell proliferation (pregnancy, infancy, chronic lymphocytic leukaemia, malignancy, psoriasis)
Drugs – alcohol, anticonvulsants
Vitamin B12 deficiency
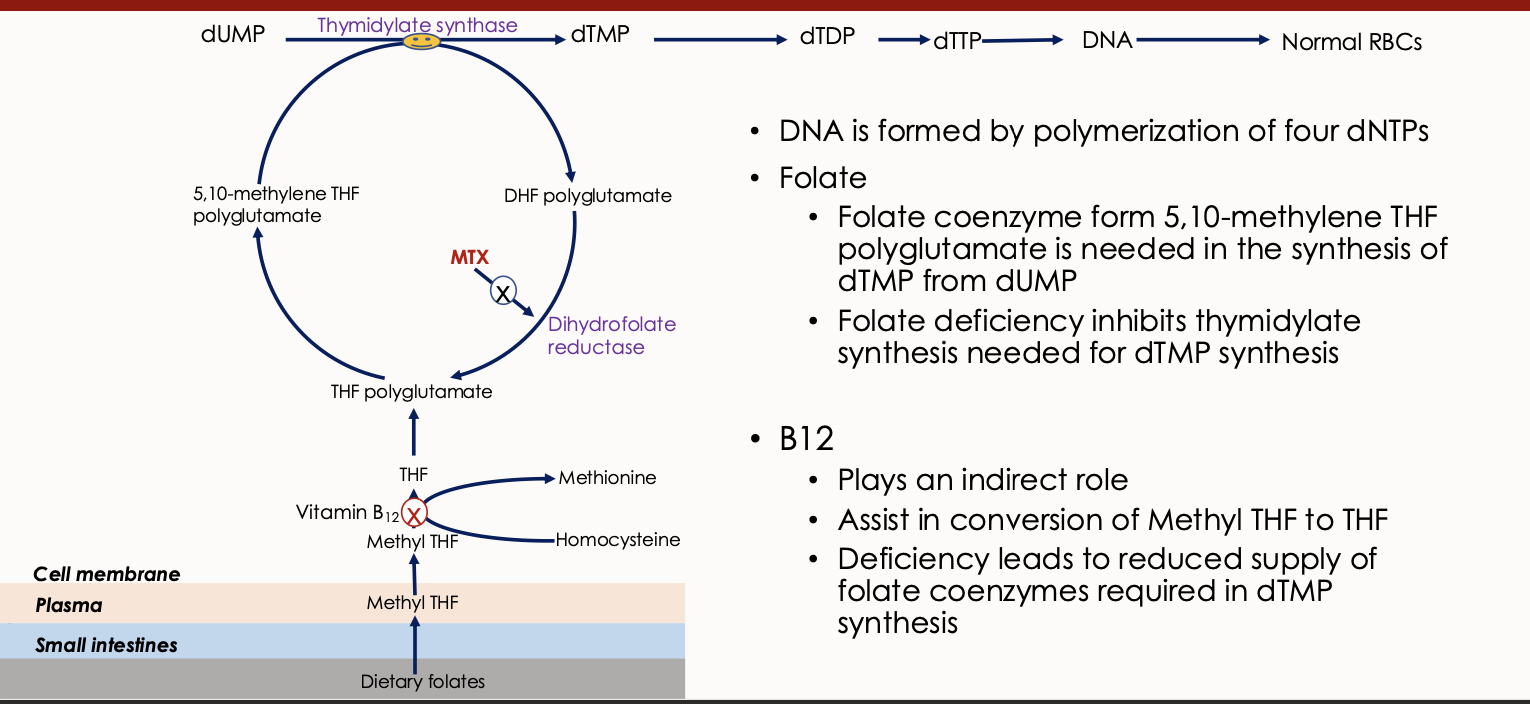
Explain what this image means