SNC2D1- Summative Topic Terms Bell Ringer
1/178
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
179 Terms
What does WHMIS stand for?
Workplace Hazardous Materials Information System
What does this symbol mean?
Biohazard (Can be infectious to body)

What does this symbol mean?
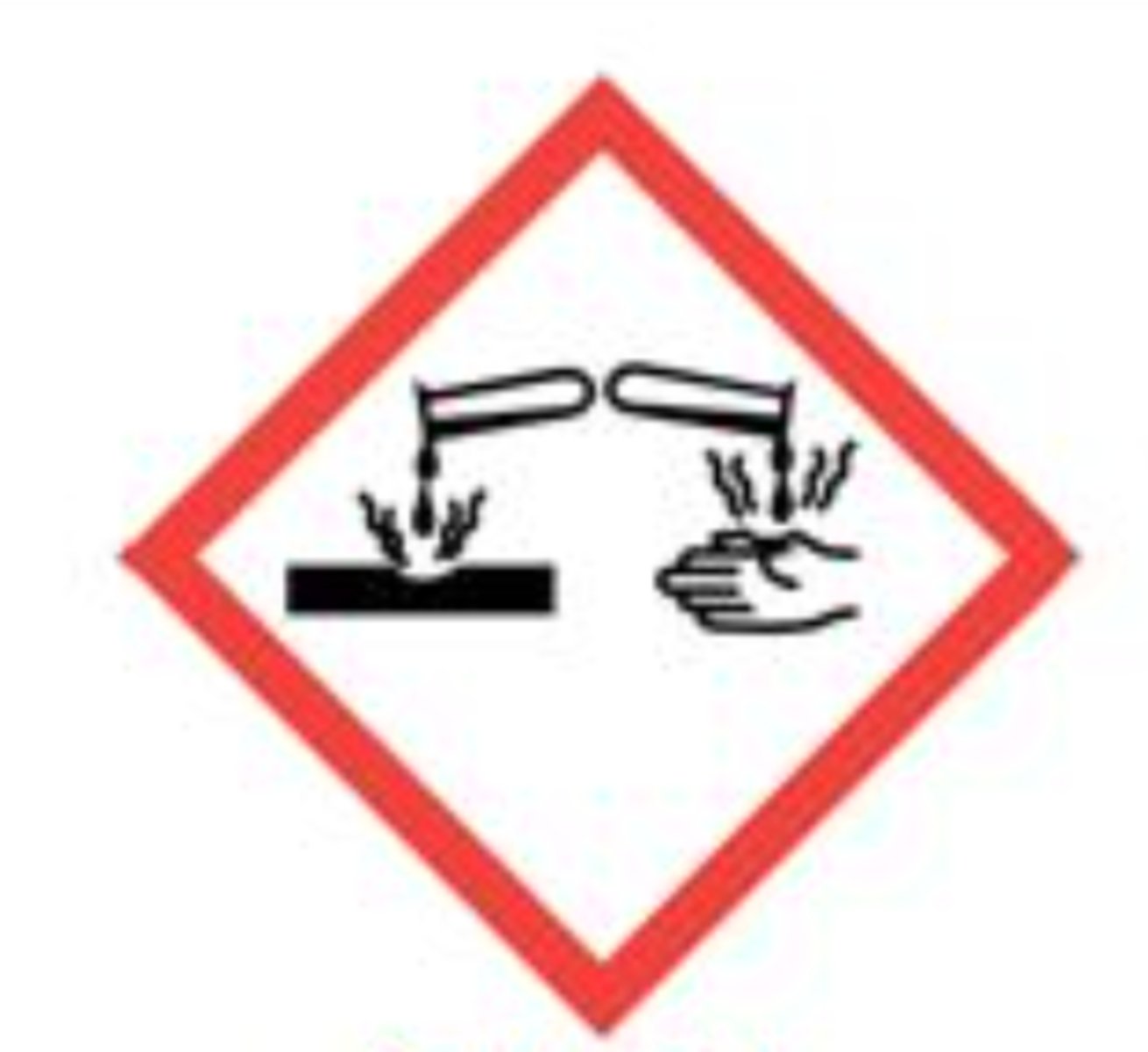
Corrosive (Can cause corrosin to skin)
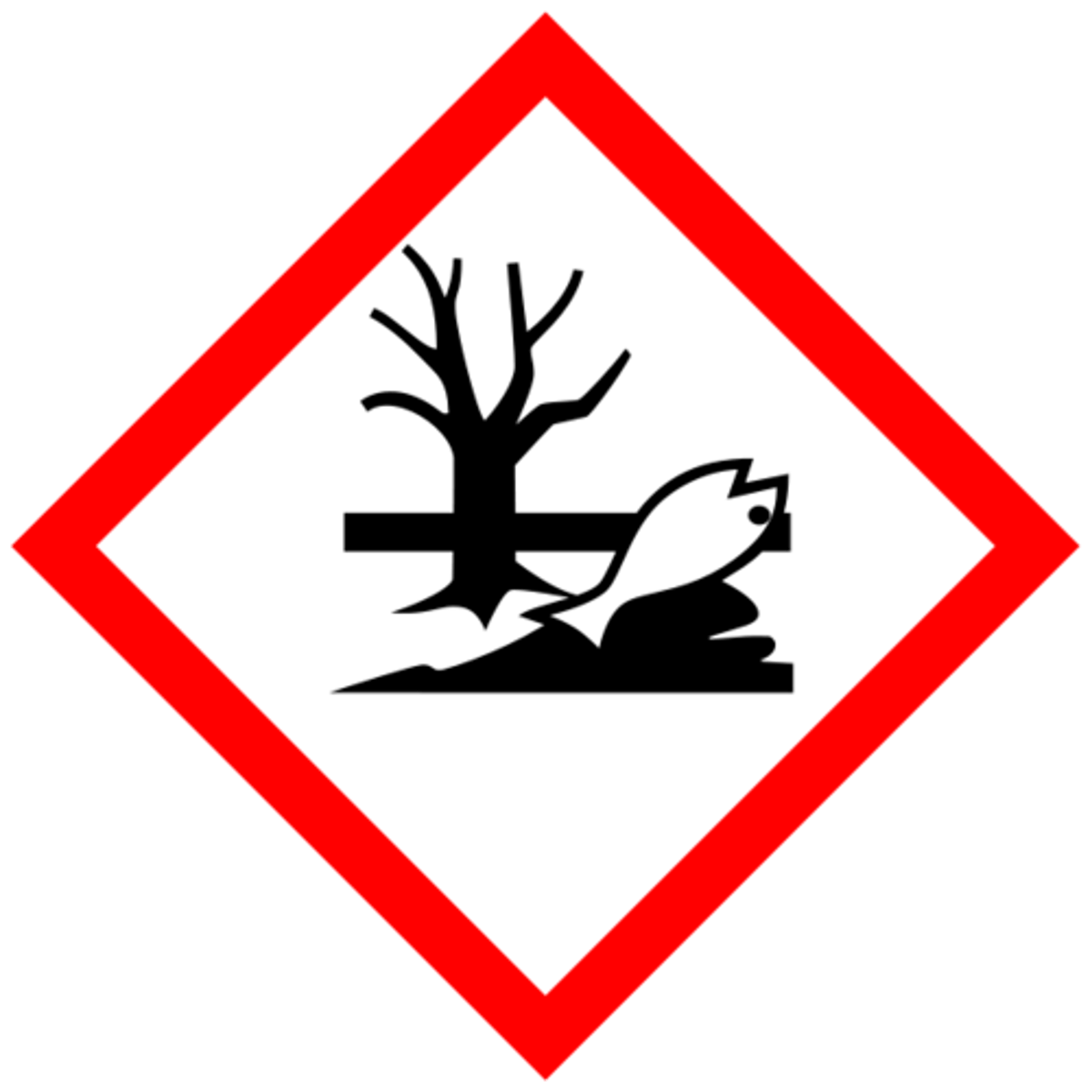
What does this symbol mean?
Envoriment (Toxic to aquatic lfe)
What does this symbol mean?
Exclamation mark (Hazardous to the ozone layer)

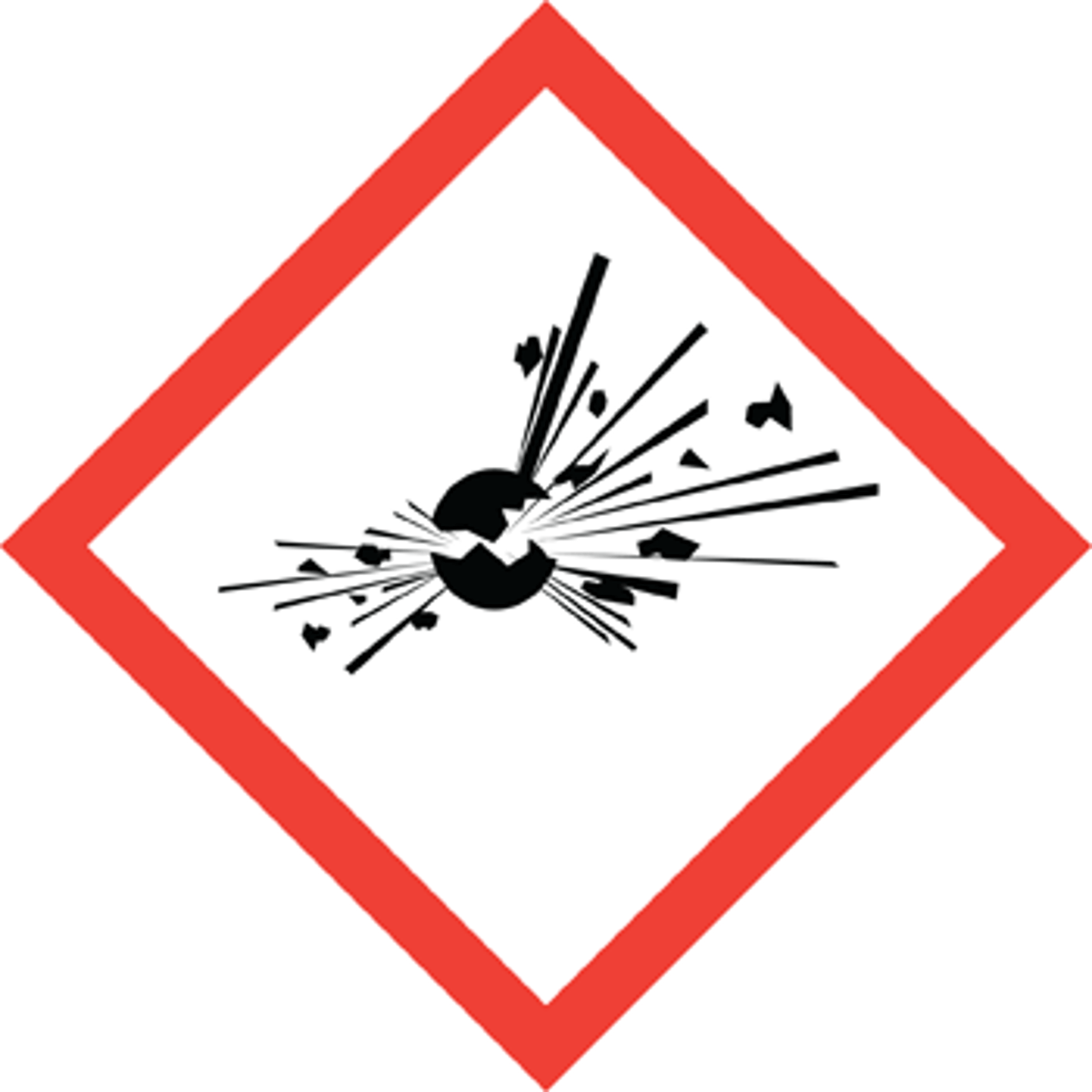
What does this symbol mean?
Exploding bomb (Reactive/Hazard)
What does this symbol mean?
Flame (Fire hazard)

What does this symbol mean?
Flame over circle (Oxidizer/ Source of oxygen)

What does this symbol mean?
Gas cylinder (Gas under pressure)

What does this symbol mean?
Health Hazard (Severse health hazard)

What does this symbol mean?
Skull and cross bones (Can cause death or toxicity)

IONS AND IONIC COMPOUNDS
IONS AND IONIC COMPOUNDS
What is the octect rule?
That all elements want to achieve full valence and become like a noble gas
What are ions?
Ions are charged atoms
What is a positive ion called?
A cation
What is a positive ion?
An atom that loses electrons and takes on a positive charge
What are negative ions called?
A anion
What are negative ions?
An atom that gains electrons and takes on a negative
What is an ionic compound?
A compound formed between a metal and a nonmetal
What happens when an ionic compound is formed?
The metal loses electrons to form a cation and the non-metal gains electrons to form an anion
NAMING
NAMING
What is the naming rule when naming ionic compounds?
You name metal element first followed by the non-metal element that has its end removed and replaced by the suffix - ide
What are used in the name of the compound to indicate which ion charge is present with multivalent metals?
Roman numerals are used (I, II, III, IV, V)
How can polyatomic ions form iconic compounds?
Polyatomic ions can combine with metals to form ionic compounds
What is a covalent bond?
Two non-metals that share electrons to achieve full valence
What are the two key things in a compound while sharing?
The lone pair which are the electrons not shared and the unpaired electrons that are shared
What are molecular compounds?
Compounds that are composed of two non-metals and they are held together by covalent bonds
How do you name molecular compounds?
In molecular compounds, you use a prefix to indicate the number of each atom present
What are the prefixes?
Mono, Di, Tri, Tentra, Penta, Hexa, Hepta, Octa, Nona, Deca
What are the Diatomic Elements (HOFBrINCl)?
H2, N2, O2, F2, Cl2, Br2, I2
What is some evidence of chemical reactions?
Change in colour, odour Formation of a gas/solid, or precipitate, Release/absorption of heat
What is some evidence of a physical change?
Change in texture, shape, temperature, and a change in the state of matter
What is the diffrence between a chemical and physical change?
A physical change in a substance doesn't actually change the substance where as a chemical reaction forms a new substance
What is a reactant?
A chemical, present at the start of a reaction, that is consumed during the reaction
What is a product?
A chemical that is created during a chemical
reaction
What is the Law of Consveration of Mass
In any given chemical reaction, the total mass
of the reactants equals the total mass of the products
What does a systhesis reaction look like?
It has 2 seperate reactants and 1 product
What does a decomposition reaction look like?
It has 1 compound reactant which become 2 seperate products
What does a single displacement reaction look like?
A + BC --> B + AC (It has 1 seperate reactant and 1 compound rectant which the seprate rectant kicks out the 1 compound and becomes a compound and the og compound becoes seperate)
What does a double displacement reaction look like?
AB + CD ---> AD + CB (Basically all the compounds switch which is a very noticable reaction)
What does a combustion reaction look like?
CH + O2---> CO2 + H2O (Usually a reaction with carbon and oxygen as products and and outcome of water and carbon dioxide)
ACIDS AND BASES
ACIDS AND BASES
What is an acid?
A compound that produces hydrogen ions ( H+ ) when it dissolves in water
What are the two types of acids?
Binary acids and Oxyacids
What are binary acids composed of?
They are composed of two elements, hydrogen and a non-metal
How do you name binary acids?
You write "hydro" then the non-metal with the suffix "ic" and then write acid
What are oxyacids composed of?
Hydrogen and a polyatomic ion that ends in O (the ones that end in ate only)
WHAT SHOULD YOU ALWAYS WRITE AFTER CREATING A FORMULA FOR AN ACID?
Aways write (aq) after the formula
How do you name oxyacids?
You name the polyatomic ion with the suffix ic and add acid after
What are bases?
A compound that produces hydroxide ions (OH-) when it dissolves in water
What are some similarities between acids and bases?
They both dissolve in water to produce ions, conduct electricity and can burn skin
What are some everyday acids?
Lemon juice, Vinegar, Aspirin, Stomach Acid
What are some everday bases?
Caffeine, Baking soda, Soap, Bleach
Taste and feel of acids? (physical properites)
They are sour and have no distinct texture
Taste and feel of bases? (physical properties)
They are bitter and have a slippery texture
Acids corrosion and reaction with metals? (chemical properties)
Corrodes metal and reacts with metals to produce hydrogen gas
Bases corrosion and reaction with metals? (chemical properties)
Does NOT corrode metals and has NO reaction with metals
What is a neutralization reaction?
It is a special kind of double displacement reaction
How do you write a neutralization reaction?
It's like double displacement but where the OH and H in the compound create H20 and the salt is formed by using the left over elements and adding them together in a compound (ykwimm)
What is a Ph scale?
A scale ranges from 0 to 14, which is used to
classify a solution as acidic, basic, or neutral
What are the ranges of numbers for acidic or basic on the scale?
pH less than 7 is acidic, pH greater than 7 is basic, pH equal to 7 is neutral
What is each decrease on the scale represent?
Each decrease of means something is 10x more acidic
What are the 3 chemical indicators that can be used to identify acid and bases?
Red litmus paper, Blue litmus paper, and Ph paper
What is another way you can test if something is an acid or base?
Using phenolpthalein
When using red litmus paper, and blue litmus paper what colours indicate an acid or base?
Red litmus- Stays red if acid, turns blue if base. Blue litmus-Stays blue if base, turns red if acid
When using phenolphthalein what colours indicate acid or base?
A base is pink and an acid is clear/says the same
What is the cell membrane?
A thin boundary covering that protects the cell from its external enviroment?
What is the function of the cell membrane?
Serves as a boundary and allows materials to pass in and out of the cell through pores by the process of diffusion
What is the smooth ER?
It extends from the rough ER to form a network of membrane tubules, and has no ribosomes on it
What is the function of the smooth ER?
To make lipids and hormones
What is the rough ER?
Transport system of the cell. It has tubules and sacs leading from the nuclear membrane and connecting to everything in the cell and contains ribosomes on the surface
What is the function of the rough ER?
Transporting and exporting proteins synthesized at the ribosomes
What are golgi bodies?
A stack of flat, bag-like structures that store and eventually release various products from the cell
What is the function of the golgi bodies?
To sort and package proteins and other molecules for transport out of the cell
What is the lysosome?
A lysosome is a vesicle that is small with round structures and contains digestive enzymes
What is the fuction of the lysosome?
To remove waste from the cell and to destroy dead cells
What is the nucleus?
The dense ball shaped organelle that directs all cell activites and contains DNA
What is the function of the nucleus?
To control all of the cells acivities
What is the mitochondria?
A bean shaped organelle located in the cytoplasm known as the powerhouse of the cell
What is the function of the mitochondria?
To create cellular respiration
What is a vacoule?
A large open storage area that is bigger in plant cells then in animals
What is the function of the vacoule?
To store water and nutrients needed by the cell
What is the cell wall?
A rigid tough wall made of cellulose found in plant cells
What is the function of a cell wall?
To protect and support the cell
What is a chloroplast?
Green structures that contain chlorophyll found in plant cells
What is the function of a chloroplast?
To trap energy from the sun to make glucose which is known as photosynthesis
MICROSCOPES
MICROSCOPES
What is the actual size of specimen measured in?
Micrometers- 1mm= 1000 micrometers
How do you calculate the total magnification of a microscope?
Ocular lens (10x) multiplied by objective lens (4x,10x,40x)
MITOSIS
MITOSIS
What are the four stages of mitosis?
Prophase, metaphase, anaphase, telophase
What is interphase?-1/2
The longest stage of the cell cycle which is divided in three parts
What is interphase?-2/2
The chromosomes start to become invisible after cytokinesis has finished then are replicated later on in the cycle
What happens in prophase?-1/2
The chromatin condenses to form paired chromosomes (sister chromatids) and the spindle is formed
What happens in prophase-2/2
Centrioles then move to each pole, the nucleolus starts to disappear and the nuclear membrane breaks down
What happens in metaphase?-1/2
Now the nuclear membrane has disappeared and the centrioles have reached opposite sides
What happens in metaphase?-2/2
Spindle fibres then attach to the centromere, and the chromosomes/chromatids line up at the equator
What happens in anaphase?-1/2
Spindle fibres then shorten and the centromeres split
What happens in anaphase?-2//2
Sister chromatids separate and move to opposite poles (sides of the cell)
What happens in telophase?-1/2
Daughter chromosomes have reached opposite poles and begin to uncoil and the nuclear membrane and nucleolus reforms