Required Practical 5 - Oxidation of Alcohols
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
State the reagents used for the oxidation of a primary alcohol.
Alcohol, acidified potassium dichromate (VI) and dilute sulfuric acid
State the conditions needed for the partial oxidation of a primary alcohol.
Excess alcohol and distillation apparatus
State the observation seen when a primary alcohol is oxidised using acidified potassium dichromate (VI).
The mixture turns from orange to green
State the safety precautions that should be taken when carrying out a distillation.
Wear gloves (oxidising agent is toxic) and concentrated sulfuric acid is corrosive
Wear goggles
Describe a method to partially oxidise propan-1-ol to pronanal.
Add about 10cm³ of dilute sulfuric acid to a round-bottomed flask
Add 3g of potassium dichromate (VI) to the acid and some anti-bumping granules
Shake until mixed
Add 1.5cm³ pronan-1-ol from a dropping pipette, shaking
Assemble the distillation apparatus
Gently heat and distil the propanal into a conical flask
Draw a diagram of the distillation apparatus.
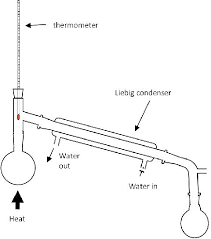
Why does the water need to flow through the condenser in a specific direction?
It must enter at the bottom and leave at the top, as it allows the condenser to fill completely so that all of the vapour can condense
Where should the bulb of the thermometer be relative to the condenser?
At the T-junction that connects the condenser
How should flammable organic reactants be heated?
In a water bath over a bunsen burner, or using an electric heater
State the conditions needed for the full oxidation of a primary alcohol.
Excess potassium dichromate (VI) and reflux apparatus
Describe a method for the full oxidation of a primary alcohol.
Measure 5cm³ water into a boiling tube and add 6g of potassium dichromate (VI)
Add 1.5cm³ pronan-1-ol into a round-bottomed flask and ass 5cm³ water and a few anti-bumping granules
Set the apparatus for reflux
Add 2cm³ concentrated sulfuric acid down the condenser and then add the oxidising agent solution
Heat using a water bath and then distil the product off using the distillation apparatus
How does a reflux apparatus work?
The condenser prevents organic vapours from leaving the mixture by condensing them back into the mixture
Why should the end of the tube be left open in reflux?
To allow gas to escape as pressure build-up can cause the apparatus to smash
Why are anti-bumping granules used?
It stops the mixture from bubbling too much