Ch 3: Atoms
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
What are the subatomic particles that atoms are composed of?
Protons, neutrons, electrons
In atoms, _______ attract and ______ repel:
opposites, identicals
What is the charge of a proton?
Positive
What is the charge of a neutron?
Neutral, no charge
What is the charge of an electron?
Negative
What two parts of an atom are found within the nucleus?
Protons and neutrons
What is the role of a proton?
To define the element using the atomic number
What is the role of neutrons?
To add mass
What is the role of electrons?
To control chemical behavior
The atomic number (Z)= the number of ______ in an atom
Protons
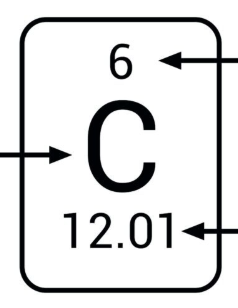
What is the overall term for the number about the chemical symbol (ex: 6)
The atomic number
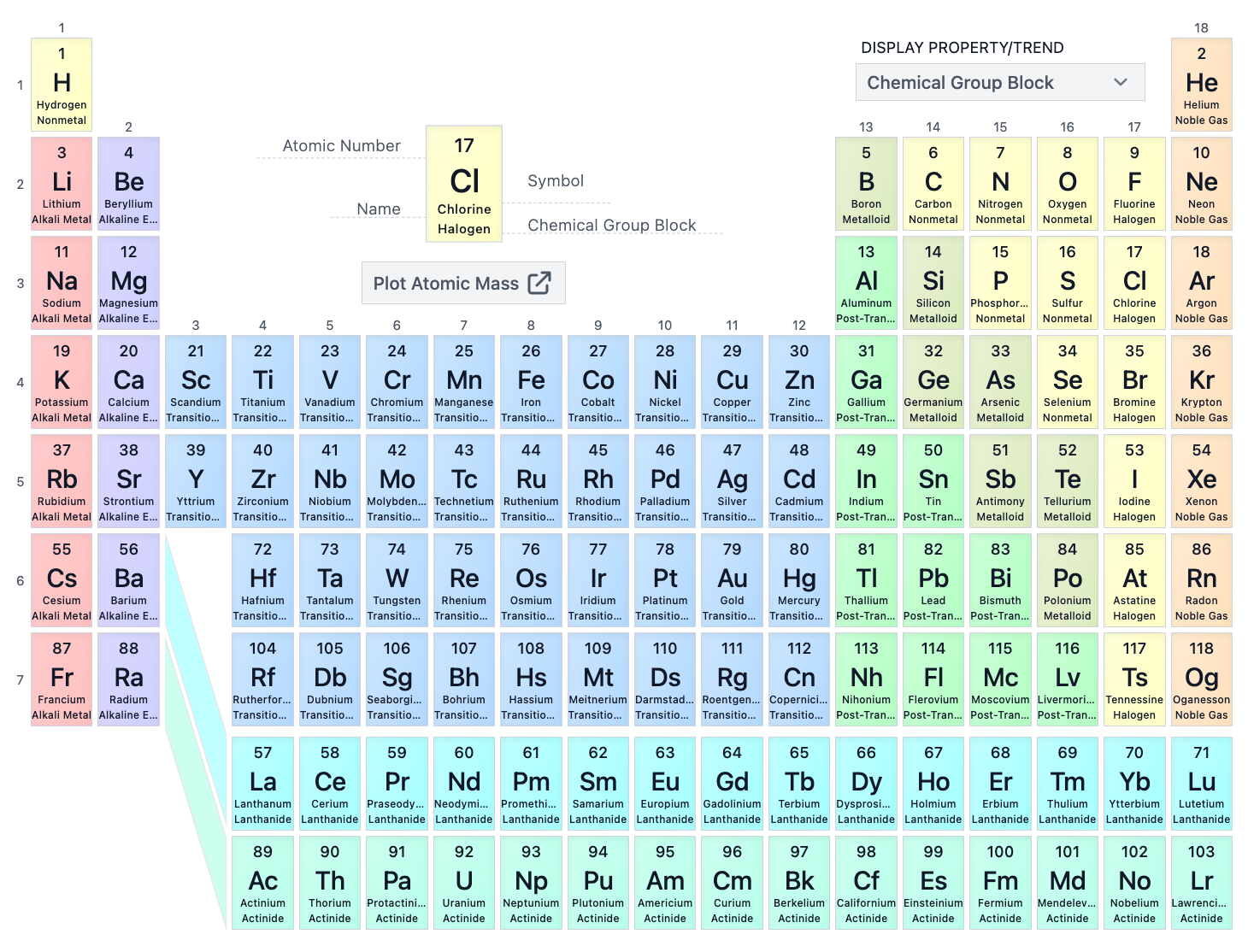
What is the atomic number of Oxygen (O)?
8
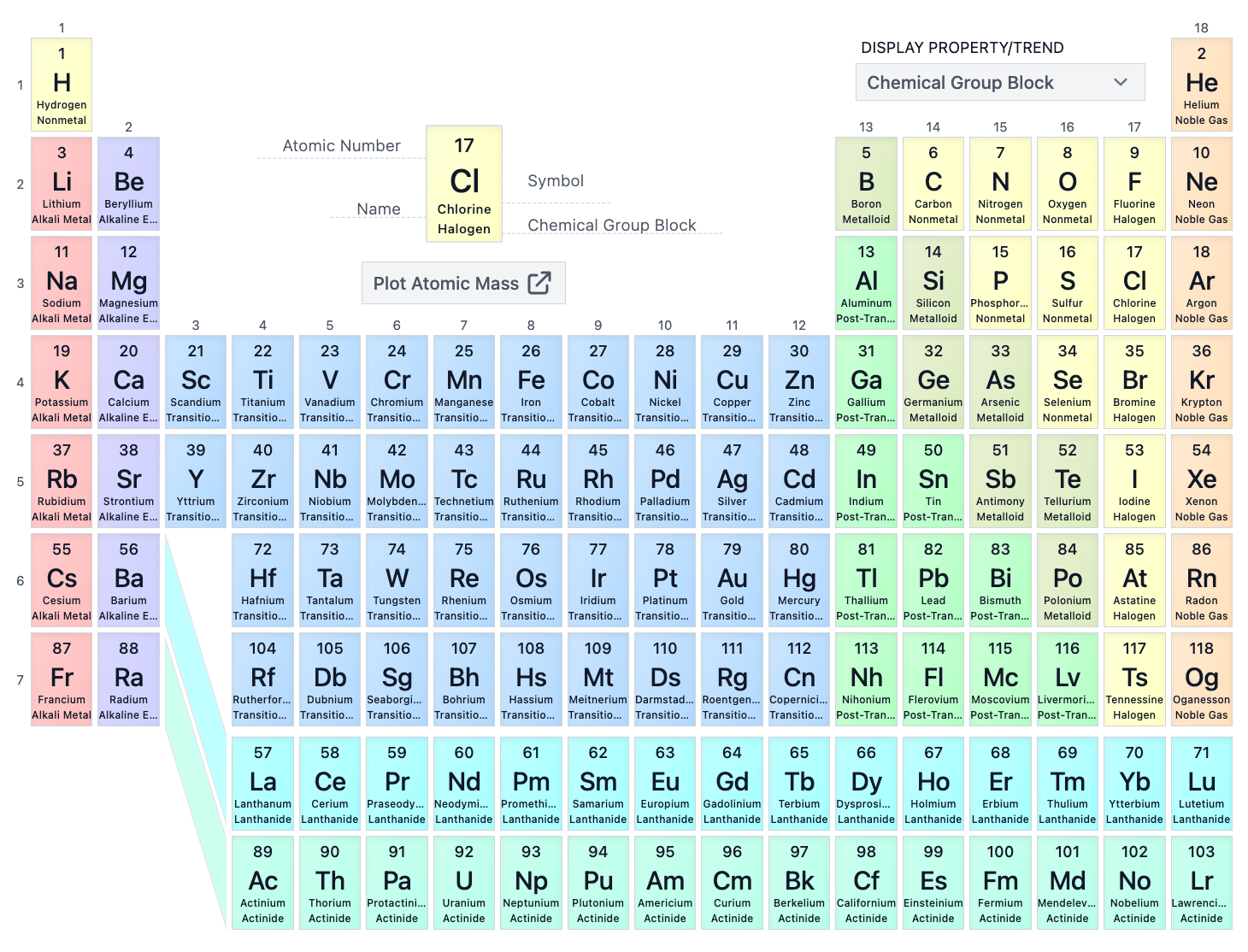
What is the atomic number of Zinc (Zn)?
30
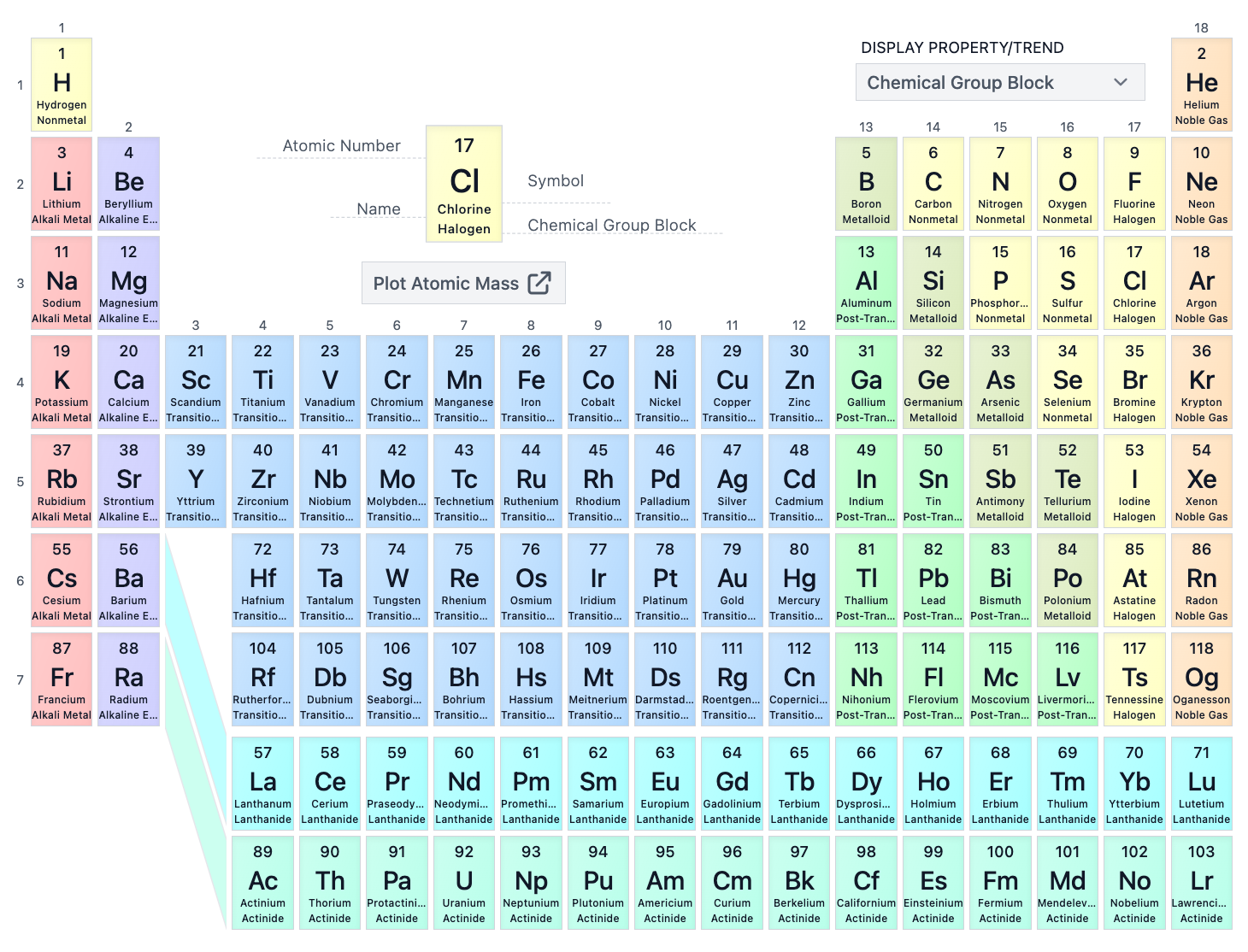
What is the atomic number of Barium (Ba)?
56
The number of protons also equals the number of ________:
Electrons
What charge do atoms initially start off as?
Neutral- Zero charge
What is the equation for finding an atom’s mass number?
Protons + Neutrons
Can you find an atoms atomic number on a periodic table?
Yes
Can you find an atoms mass number on a periodic table?
No
Can you find an atom’s number of neutrons on a periodic table?
No
What is the equation to finding the amount of neutrons an atom has?
Mass number - atomic number (protons)
Atomic mass and mass number are NOT the same! Atomic mass is a _______ and mass number is a ______ number:
Decimal; Whole
If a carbon atom has 6 protons and 6 neutrons, what is its mass number?
12
If an oxygen atom has 8 protons and 10 neutrons, what is its mass number?
18
If a chlorine atom has 17 protons and 18 neutrons, what is its mass number?
35
An atom has a mass number of 40 and 20 protons. How many neutrons does it have?
20
An atom has a mass number of 56 and 26 protons. How many neutrons are present?
30
An atom has 19 protons and a mass number of 39. How many neutrons does it have?
20 neutrons
Nitrogen gas is used in scuba diving tanks. How many protons and electrons does an electrically neutral atom of nitrogen (N) have?
7 protons, 7 neutrons
What is an isotope?
Atoms of the same element (same number of protons) but different number of neutrons
Isotopes have the same _______ number, but different ______ numbers:
atomic; mass
The weighted average of the mass of all the naturally occuring isotopes of an element is the:
Atomic mass
The “rings” around the nucleus of an atom are specifically called:
Energy levels (n)
n=1, n=2, n=3, n=4, and n=5 are called…
Electron energy levels
Within each energy level are sublevels identified with the letters…
s, p, d and f
What is the atom’s outermost energy level called?
Valence shell
What is important for how atoms will react or bond with other atoms?
Valence electrons
What can be used to determine how many valence electrons an element has?
The elements group number on the periodic table
How many valence electrons are found in the elements oxygen (O) and sulfur (S) respectively?
both have 6 valence electrons
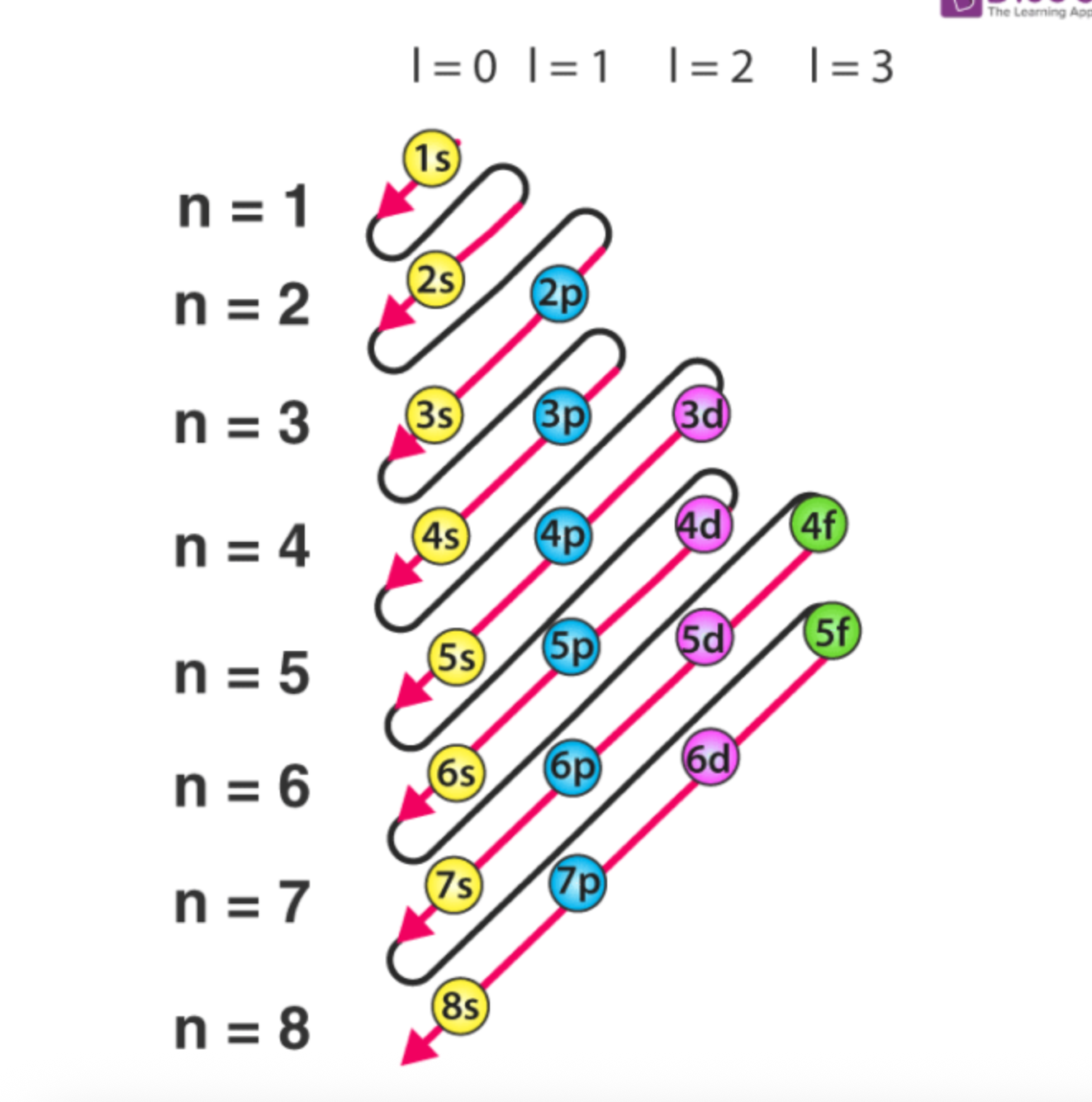
What is this diagram used to solve for?
Electron configurations
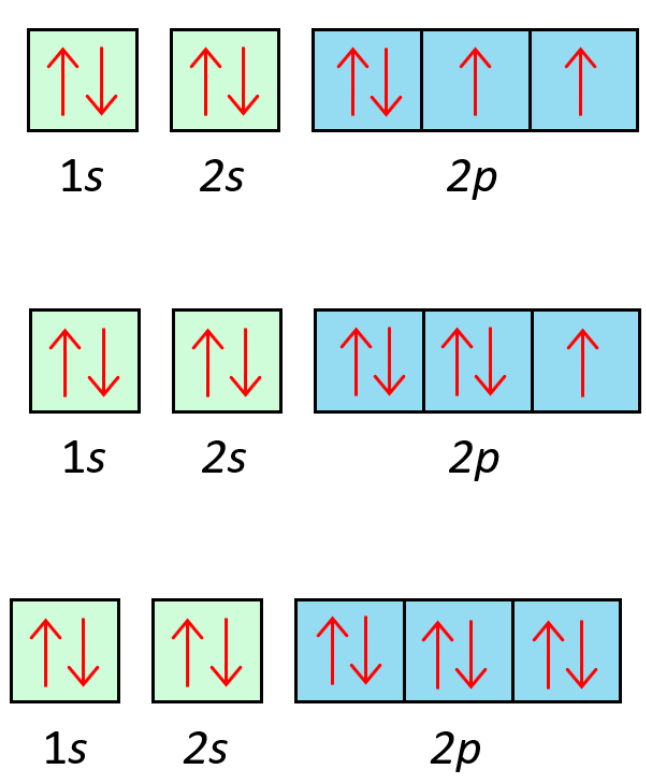
What do these represent?
Orbital diagrams
What kind of symbols are used to represent valence electrons?
Electron-dot symbols
How many electrons can each side of a chemical symbol hold?
2
Electrons fill the ______ energy orbitals first:
s <p < d < f
lowest
How many electrons can each orbital box hold?
Two
What directions must the electrons in the same orbital box be in?
Opposite
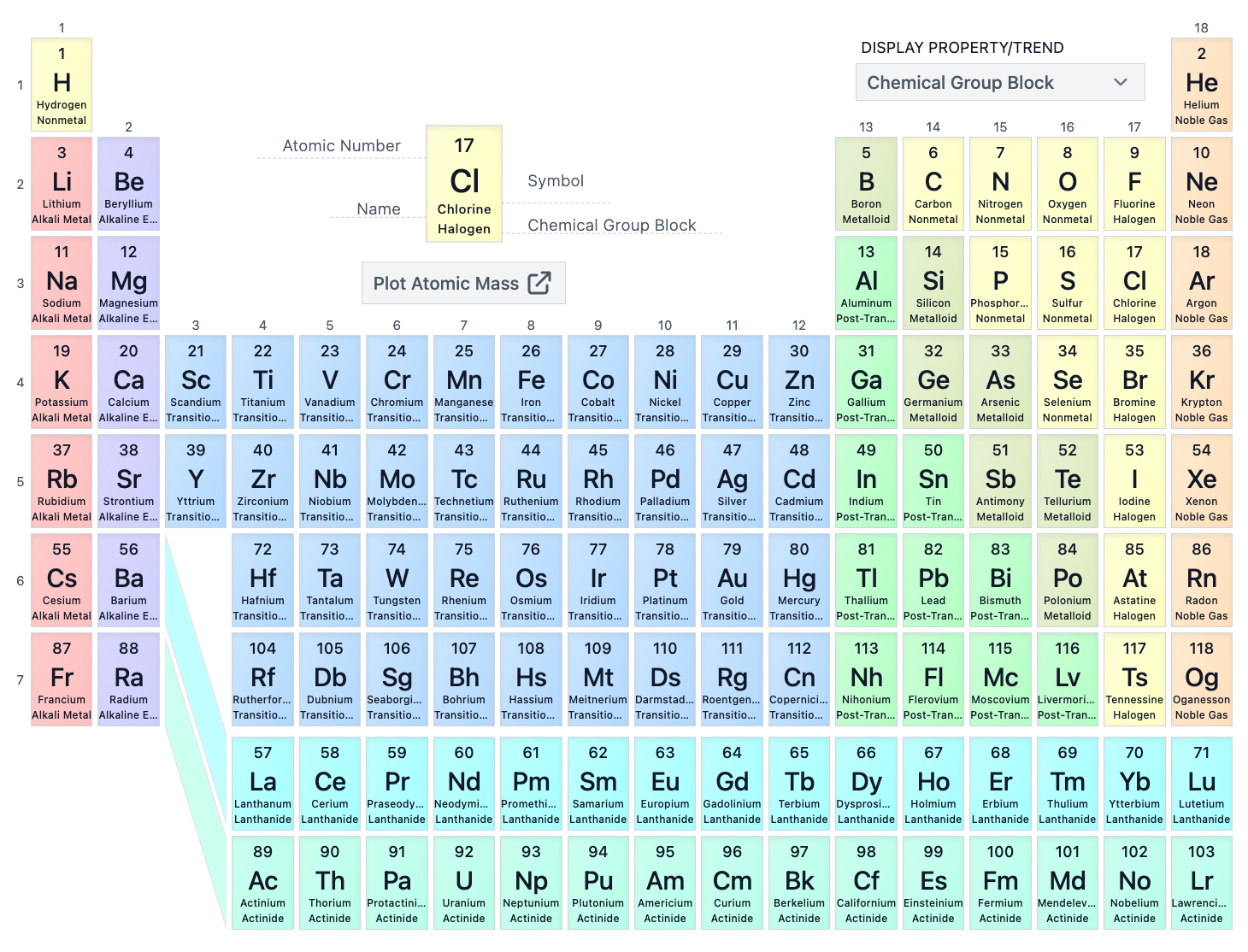
The S-block consists of which groups on the periodic table?
Groups 1A, 2A and Helium
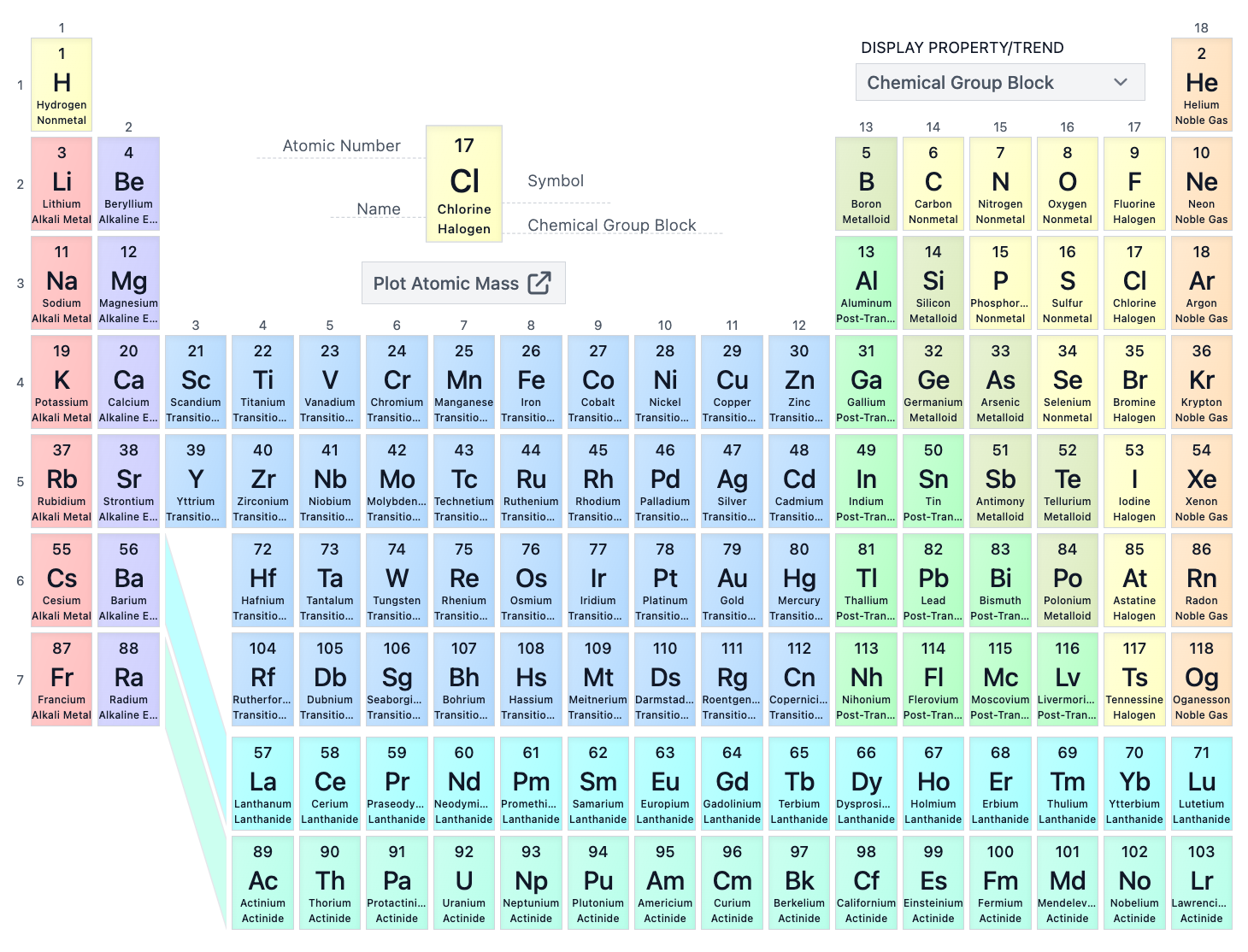
The P-block consists of which groups on the periodic table?
Groups 3A-8A
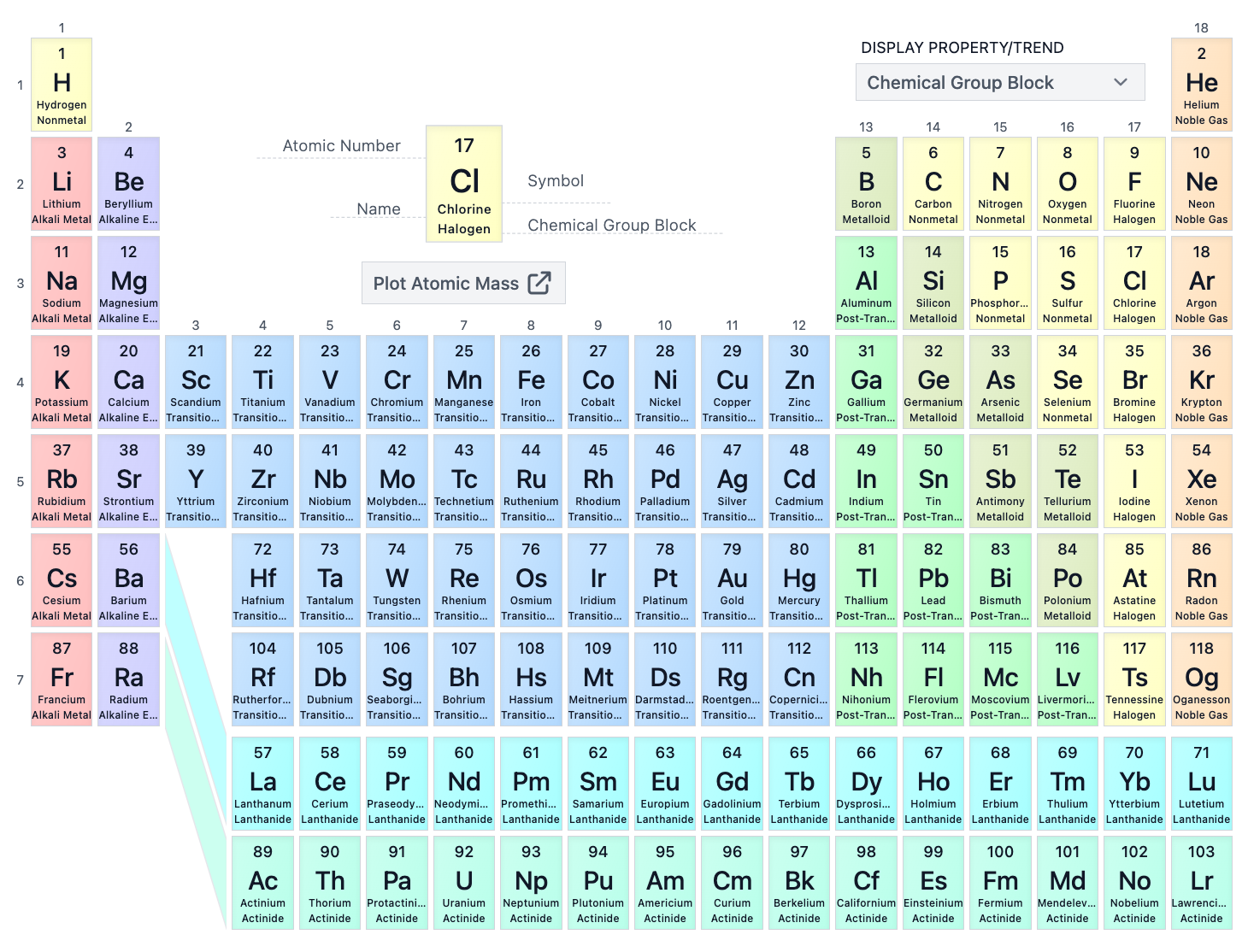
The D-block consists of which groups on the periodic table?
10 columns of transition metals
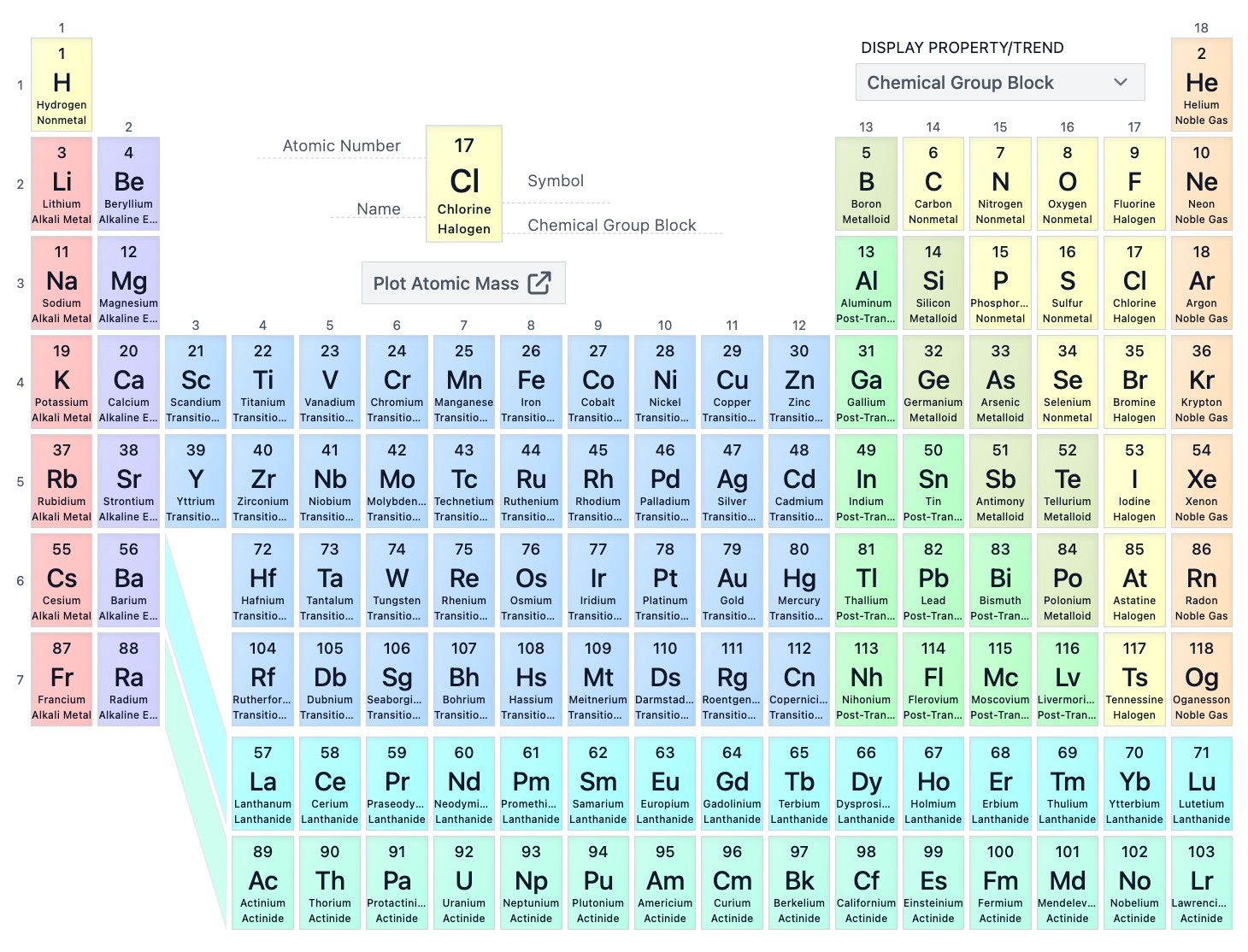
The F-block consists of which groups on the periodic table?
the two bottom group of inner transition metals
What is the electron configuration of oxygen?
1s², 2s², 2p^4
What is the abbreviated electron configuration for oxygen?
[He] 2s², 2p^4