BIOL 4106 - Exam 3
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
75 Terms
Scolex
The anterior end of the tapeworm
Equipped with a holdfast organ: suckers, hooks, grooves
Types of suckers:
Acetabula: 4 cup-shaped with a cup-shaped muscular wall
Bothria: 2 “slit-like“ shallow pits/grooves
Rostellum
Protruding dome-shaped area on the most anterior end of scolex
Cestode Neck
The region between scolex and strobila segments
Contains stem cells that give rise to new proglottids (Praziquantel)
Cestode Strobila
Long chain of proglottids (segments)
Each has sets of reproductive organs of both sexes
Strobilization
Growth of strobila
Proglottids closet to scolex are most immature
The more posterior, the more mature
Gravid Proglottids
“Gravid“ = Filled with eggs
Proglottids “cross fertilize“
How Proglottids are Shed
Intact gravid proglottid – [Taenia spp]
detaches and shed intact in feces
Proglottid disintegrates as shed and eggs go out with feces – [Hymenolepsis spp]
Eggs shed from attached proglottid through uterine pore – [Diphyllobothrium spp]
senile (empty) proglottids detach in a short chain which is shed in feces
Cestode Tegument (Cuticle)
Absorption: All nutrients absorbed through the tegument
Covered in microvilli called “microtriches” even on the sucker
Increases the absorptive area of the tegument
Excretion of wastes
Osmoregulation (water balance)
Monecious
Cestodes = Hermaphroditic
Sperm are transferred between mature proglottids that lie next to each other
Dipylidium Egg
Thin shell
Operculated in [Diphyllobothrium latum]
In egg packets for [Dipylidium caninum]
Diphyllobothrium sp, Dipylidium caninum, Hymenolepsis sp
Taenia Egg
Thick striated shell
Hexacanth embryo within
Taenia sp and Echinococcus sp
Cestode Life Cycle
Egg → Onchosphere → Metacestode → Adult
Onchosphere: Hexacanth larva in egg
Metacestode: Juvenile (larval form of tapeworm)
IH: Herbivore
DH: Carnivore
Hymenolepsis nana doesn’t follow this; only needs one host
Pseudophyllidan Life Cycle
Egg → Onchosphere = Coracidium → Procercoid → Plerocercoid → Adult
Coracidium: Ciliated free-swimming oncosphere, has hooks
Procercoid (1st IH):
Usually in copepod
First metacestode stage
Plerocercoids (2nd IH):
Fish will eat 1st IH
Procercoid → Penetrates gut wall → muscle → Plerocercoids
Infective Stage for DH
Sparganum
Term for plerocercoid of Diphyllobothrium (Spirometra) mansonoides
What is difference between procercoid and plerocercoid?
Plerocercoid develops a scolex and has some strobilia formation (a true juvenile cestode)
Cyclophyllidian Life Cycle
Egg → Onchosphere → Metacestode → Adult
Egg has to be eaten by IH
Eggs can be viable in soil for a while
Onchosphere uses hooks penetrate thru gut wall → tissues → metacestode
4 Basic Metacestode Forms in Cyclophyllidians
1. Cysticercoid
Solid cyst with a single, inverted scolex within
IH is an invertebrate
[Dipylidium sp] and [Hymenolepsis sp]
2. Cysticercus (“bladder worm”)
Fluid-filled cyst (“bladder” worm) with a scolex inverted and invaginated
IH is a vertebrate
[Taenia sp]
3. Coenurus
Fluid-filled cyst with several inverted scolices, each on a stalk
IH is usually a vertebrate
[Multiceps sp]
4. Hydatid Cyst
IH is a vertebrate
Grows slowly but can get very large containing quarts of antigenic fluid with 1000s of scolices
Cyst is lined with germinal epithelium which gives rise to:
Individual protoscolices (scolex without bladder)
Daughter capsules (each with many protoscolices within)
“Hydatid Sand” – a granular deposit in hydatid cysts consisting of liberated daughter capsules and free protoscolices
Asexual reproduction is often referred to as “budding” from the cyst
Two Types of Hydatid Cysts
1) Unilocular: Seen in [Echinococcus granulosus] infections
2) Multilocular: Daughter capsules bud externally
As daughter cysts bud outward, the multilocular cyst invades the tissues of infected organs
Makes this form highly invasive like an aggressive metastatic tumor as parasite tissue replaces host organ tissue
Seen in [E. multilocularis] infections
Adult Tapeworms in DH
Metacestode form excysts in the gut
Hymenolepsis diminuta can increase its size to over a million times greater than the initial size
Once mature size for species is attained, growth then becomes the production of proglottids to replace the gravid ones that are shed
No real pathology associated with adults in the intestinal lumen attaching to the gut lining
Absorb nutrients from digested nutrients in the lumen provided by the host
Cestodes eat when you eat!
Pseudophyllidae
[D. latum] & [D. mansonoides]
Scolex with bothria
Proglottids remain attached and shed eggs through pore
Genital pores are central
Eggs are operculated (look like trematode eggs)
1st IH: Crustacean – copepod
2nd IH: Fish
DH: Can be a human
Diphyllobothrium latum
Distribution: Worldwide
1st IH: Copepod (Procercoid Stage)
Eggs in FW → Coracidium → Eaten by copepod
2nd IH: FW fish; minnow/salmon (Plerocerocid Stage)
FW fish can eat FW fish many times and stay as plerocercoid; must be eaten by DH to → Adult
DH: “Fish-eating“ carnivores
Transmission: Eating raw FW fish containing plerocercoids
Niche: SI
No pathology unless the person already has anemia → worsens Vitamin B12 anemia deficiency
Morphology: Large worm
Proglottids are wider than longer
Eggs: operculated
Dx: Long strings of exhausted (senile/empty) proglottids in feces
Prevention: Don’t eat raw FW fish (salmon)
Diphyllobothrium mansonoides (Spirometra mansonoides)
1st IH: Copepods
2nd IH: Any vertebrates (NOT FISH)
Can get infected by procercoids and plerocercoids
Infection is “Sparganosis”
Painful nodules that can go to eye → brain
Humans are accidental 2nd IH
Drink water with infected copepods
Eating raw 2nd IH
Applying IH skin on a lesion (East Asia)
Frog or snake skin on wounds to heal
DH: Cats or Dogs only
No treatment (;-;)
Prevention: Don’t eat raw or undercooked meat (snake) and don’t wear frog skins
Taenia saginata
“Beef Tapeworm“
Distribution: Anywhere that consumes beef and lack of sanitation
IH: Cattle (herbivore)
Egg with hexacanth → Eaten by cattle → Hatch in SI → Hexacanth uses hooks and penetrates gut wall → enters the bloodstream → striated skeletal muscle tissue → develops into cysticercus (measly beef) → Eaten by human
DH: Man
Scolex everts from cysticercus → SI wall
Usually asymptomatic
Niche: SI
Morphology:
No rostellum
4 acetabular
Immature and mature proglottids are slightly wider than longer
Gravids are much longer than wide
Lateral genital pore
Dx:
Prevention: Freezing and cooking
Taenia solium
“Pork Tapeworm”
Distribution: SE Asia, Mexico, South and Central America, Eastern Europe, Micronesia, Philippines
IH: Pig and Human
Infective stage: Eggs containing hexacanth
Can cause Cysticercosis → Space occupying lesion → Neurocysticercosis
Parasites dying 2 years later will release antigens and cause pathology
DH: Human
Infective Stage: Undercooked pork (measly pork) containing cysticercus → Adults in SI → Eggs in feces
Usually asymptomatic
Morphology: Gravid proglottid is longer than wide
Dx: Proglottids
Neurocysticercosis: CAT scans, MRI, antigen capture ELISA using CSF fluid
Prevention: Don’t eat measly pork or feces
Taenia pisiformis
IH: Rodents and Rabbits
Larval predilection site for cysticercus: Peritoneum of rodent/rabbit
DH: Dog and cats only
Not infective for humans
Morphology:
Scolex has 4 suckers and a double row of hooks on the rostellum
Segments are more rectangular
Genital pores occur in irregular alternating sequences on either lateral margin
[Dipylidium caninum] is also a most common tapeworm of dogs
Echinococcus granulosus
Distribution: Europe, Africa, New Zealand, SW USA, Asia (Russia), South America, Canadian Arctic (Inuits)
The northern variety in Canada involves caribou and moose as IH
IH: Grazing herbivores or humans
Humans get hydatid cysts from ingesting eggs in dog feces
DH: Dogs and other Carnivores
Life Cycle:
Eggs with hexacanth oncosphere → IH → Hatches in SI → Pentrates gut wall → Bloodstream → Organs → Hydatid Cysts
The liver is most common
Morphology: Adult tapeworm
Smallest tapeworm
Scolex has 4 suckers and armed rostellum with hooks
Composed of scolex, neck, and 3 proglottids
Gravid proglottid disintegrates and eggs pass out with feces
Dx: NEVER BIOPSY
Radiographs, CAT scans, and MRI
Case history: dogs/sheep or travel to an endemic region
Prevention:
Keep sheep dogs dewormed
Don’t feed raw infected meat to dogs!!
Cyclophyllidae
[Taenia sp.], [Dipylidium sp.], [Hymenolepsis sp.], [Echinococcus sp.]
Scolex with 4 acetabula (suckers)
No rostellum: T. saginata & H. diminuta
Rostellum with hooks (armed rostellum)
Genital pores lateral
Eggs with thick, striated shells and containing a hexacanth embryo
Echinococcus multilocularis
IH: Rodent such as field mice, vole (and humans)
Trappers and fur handlers get it from eggs on fur or feces
DH: Wild carnivores such as foxes and other wild canids
Forms multilocular/alveolar hydatid cysts that create an aggressive, progressive, and destructive invasion of tissue
Replaces normal tissue with parasite tissue
Rx: No effective treatment; surgery
Hymenolepsis nana
“Dwarf Tapeworm“
Distribution: Cosmopolitan parasite
IH: Arthropods such as grain beetle (OPTIONAL)
DH: Rodents and Humans
Life Cycle:
Humans as DH = Ingest grain beetle with cysticercoid → Scolex everts → Intestinal wall → Adult
Humans as IH = Eggs ingested → Onchospheres hatch in duodenum → Penetrates gut wall → Forms cysticercoid → Emerges from host tissue into gut lumen → Adult
Morphology:
Adults:
Scolex with retractable armed rostellum
Genital pores unilateral
Gravid segment disintegrates and eggs pass out with feces
Eggs:
Thin outer membrane and thick inner membrane
Polar filaments on the inner membrane
Dx: Eggs in feces
Prevention: Get rid of rodents
Hymenolepsis diminuta
Distribution: Worldwide
IH: Grain beetle
DH: Rat and humans
Morphology: Larger than H. nata
Adults: Unarmed rostellum
Eggs: No polar filaments (not cloudy)
Dx: Eggs in feces
Dipylidium caninum
“Cucumber Tapeworm“
Distribution: Worldwide
IH: Flea
DH: Dogs and sometimes Humans
Humans get it from ingesting fleas with cysticercoid
No clinical disease
Life Cycle: Egg with hexacanth onchosphere → Flea → Cysticercoid → Dog/Humans → Cysticercoid → SI → Adults
Morphology:
Adult:
Scolex with 4 suckers
Armed retractable rostellum
Proglottid long with double genital pores (bilateral)
Gravid proglottid shed intact
Proglottids can crawl out the anus and onto clothing or bedding
Egg: Contained in packets of 8-15 eggs within proglottids
General Nematode Morphology
Bilaterally symmetrical
Usually tapered at both ends
External cuticle
Protective covering
Alae = lateral thickenings
Nematode Reproduction
Dioecious
Nematode Infective Stade for DH
J3 for most nematodes
Some species: Egg containing infective J3 stage
Two exceptions: Ascarids, Trichinella
Some species: J1’s hatch from egg, molt 2x → J3 stage (infective stage)
Nematode Life Cycle
Consists of 4 juvenile stages and an adult stage
Egg containing J1 → J2 → J3 → J4 → Adult
Stages are separated by ecdysis (molting) of cuticle
Hypobiosis
A developmental arrest in the parasite cycle
Development in the host will stop until certain conditions occur that are conducive to parasite survival
Oxyurida
[Enterobius vermicularis]
Cephalic alae
Medium to small worms with very pointed tails
Thin-shelled ovoid eggs that are flattened on one side
Direct life cycle
Ascaridida
[Ascaris lumbricoides] [Toxocara canis], [Toxocara cati], [Baylisascaris procyonis]
3 prominent lips
Direct life cycle
Eggs thick shelled, rough coated and very environmentally resistant
Strongylida
[Necator americanus], [Ancyclostoma duodenale], [Ancyclostoma caninum], [Ancylostoma braziliensis]
Long slender worms
Well-developed copulatory bursa in males
Usually oviparous (Lay eggs directly)
Eggs thin-shelled (release in morula stage – not embryonated yet)
Rhabditida
[Strongyloides stercoralis] & [Strongyloides fulleborni]
Small worms
Buccal cavity is small
Filariform esophagus – J3 (must become parasitic to survive)
Rhabditiform esophagus – free-living stages
Tail conical in both sexes
Trichurida
[Trichuris sp] & [Trichinella sp]
Anterior end is more slender than posterior
Buccal cavity reduced or absent
Stichosome esophagus
Eggs with polar plugs [Trichuris sp]
Enterobius vermicularis
“Pinworm“
Distribution: Temperate regions
DH: Humans (direct life cycle)
Most common in elementary or daycare
Niche: Large Intestine and Rectum
Transmission: Ingestion or inhalation
Morphology:
Adult = cephalic alae & bulbed esophagus
Crawl out at night and lay eggs → Worm dies
Eggs = Thin-shelled and flat on one side
Can be airborne
Reinfection it possible as J3 hatch and crawl back into rectum
Disease: Intense itching in perineal regions
Dx: “Scotch Tape” test
First thing in the morning immediately
Ascaris lumbricoides
“Human roundworm“
Distribution: Worldwide, more in tropical rural areas with low sanitation
DH: Humans (direct life cycle)
Niche: Upper small intestine
Does not attach to host tissue
Life Cycle:
Eggs in feces → J1 → J2 while in egg → Soil → Ingestion → Small intestine (J3) → Liver → Lungs (J4) → Cough into Stomach (J4) → SI (J5)
Morphology:
Adults = Huge with 3 distinct lips on the cephalic end
Female: Straight tail
Male: Curled tail
Eggs = Thick-shelled with a rough, bumpy surface
Can survive forever and chemical-resistant
Conditions need to be 37ºC, pH 7, low oxygen, and high CO2 to hatch
Dx: Eggs in feces
Ascaris Migratory Phase
Causes most host response
Molting substances very antigenic
Eosinophilia and increased levels of IgE
Ascaris Intestinal Phase
Few symptoms
Can obstruct the intestine or biliary, crowding effect, perforate intestine (peritonitis)
In children: stunting in growth, malabsorption, and intestinal blockage
Toxocara canis
Cosmopolitan ascarid of dogs and other canids
Life cycle in dog like A. lumbricoides in man with migratory phase from gut to liver to lung and back to gut
Can be transmitted to puppies two additional ways:
J3’s transplacentally transmitted from the infected mother to fetus
J3’s via a transmammary route from infected mother to nursing puppies
Can cause Visceral Larval Migrans and Ocular Lavaral Migrans
Toxocara cati
Roundworm of cats
Life cycle similar to Toxacara canis, except no transplacental transmission
Can cause Visceral Larval Migrans and Ocular Lavaral Migrans
Visceral Larval Migrans, Ocular Larval Migrans
Ingestion of eggs → J3 in bloodstream → Inflammation and granulomas in affected organs
CNS, liver, lungs, and eyes most seriously affected
Children are usually affected more
Ocular: Retinal granulomas → Detachment of retina → Blindness
Dx: Immunological tests (ELISA) for J2 antigens
Baylisascaris procyonis
Raccoon ascarid 🦝
Dogs can develop patent infections as definitive hosts
Increases the risk of spreading to humans
Has a liking for CNS in aberrant (wrong) host
Eggs are as resistant as ascarid eggs
Small children are most often infected
CNS signs are indicative of an eosinophilic meningoencephalitis
Rx: None
Usually fatal in humans, especially small children
Distribution:
1st IH:
2nd IH:
DH:
Morphology:
Dx:
Prevention:
New World Hookworm
Necator americanus
Old World Hookworm
Ancyslostoma duodenale
Canine Hookworm
Ancylostoma caninum/braziliensis
Hookworm
Distribution: Worldwide
DH: Humans or canine
Niche: Small Intestine (mucosa and blood)
Anemia, iron deficiency, impaired physical and mental development, low birth weight/fetal development
Life Cycle: Direct
J3 penetrates directly into skin → Bloodstream → Lungs → Small Intestine (J4) → Adult
J3 in soil are filariform larvae
Morphology:
Adults
“Hooked” anterior end
Club esophagus
Copulatory bursa
Eggs
Thin shelled
Unembryonated in fresh feces
Clinical Disease: “Ground itch/dew itch“
Dermatitis is caused by repeated infections that are very pruritic (itchy)
Infantile ancylostomiasis: Transplacental and transmammary transmission
Severe anemia, dysentery (black), fail to thrive
Dx: Eggs in feces
Prevention: Sanitation, education, wear shoes
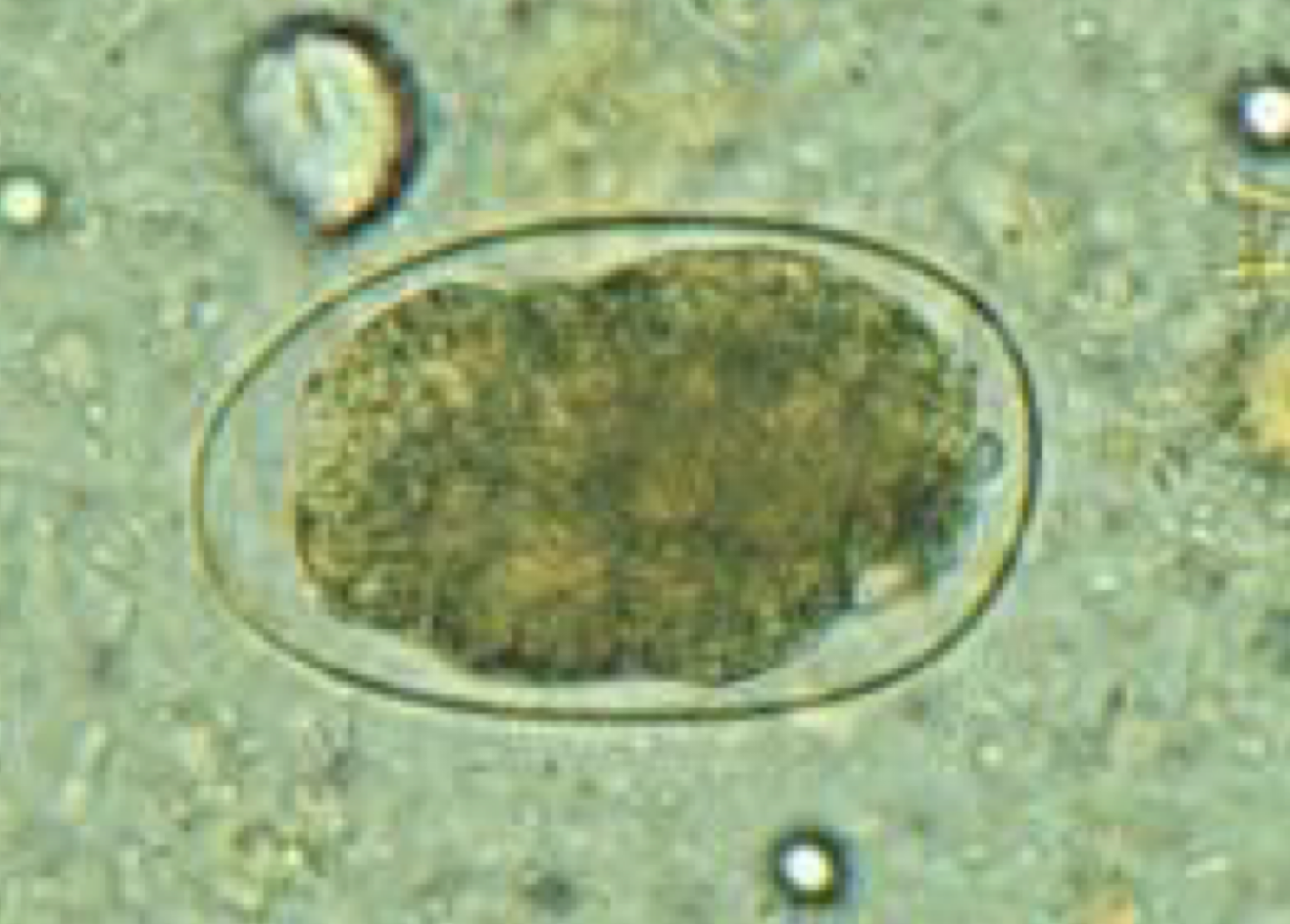
Difference between Ancylostoma duodenale & Necator americanus
Ancylostoma duodenale
Morphology: Cutting teeth, larger worm
Pathology: More pathology, more blood loss, produce more eggs, hypobiosis (arrest development)
Transmission:
Skin penetration of J3
Oral ingestion
Transplacental of J3
Transmammary of J3 (vertical transmission)
Necator americanus
Morphology: Cutting plates
Pathology: Same but not as much (no hypobiosis)
Transmission: Skin penetration
Cutaneous Larval Migrans (CLM)
From J3 of dog or cat hookworms
Creates “creeping eruption” that’s very pruritic
Scratching can cause bacterial infection
[Ancylostoma braziliense] & [Ancylostoma caninum]
Strongyloides stercoralis
Distribution: Worldwide
DH: Dogs, cats, and humans
Infective stage: Filariform J3
Life Cycle: Heterogonic OR Homogonic
Transmission: Skin penetration, ingestion from soil or water, autoinfection, transmammary
Autoinfection: J1 in gut long enough to be J3 and enter bloodstream
Compromised people get hyper infection
Given corticosteroids → Immunosupressed → Speeds molting process
Morphology:
Adults are very small
NO eggs
Only females can be parasitic
Pathology: Adult females in intestine
Local inflammation
Heavy infection: Watery, mucoid diarrhea
Dx: J1 in stool
Heterogonic Life Cycle
Free-living phase
J1 →→ Adults (male and female) → Embryonated eggs → J1 →→ J3
J3 will continue to become an adult
Homogonic Life Cycle
J1 →→ Adults (male and female) → Embryonated eggs → J1 →→ J3
A female J3 will become a filariform J3 → Skin → Bloodstream → Lungs (J4) → Esophagus → SI → Adults (J5) in gut mucosa → Eggs in gut lumen → J1 in feces
Strongyloides fulleborni
Distribution: Papua New Guinea and Africa
DH: Humans
Pathology: “Swollen Belly Syndrome“ (SBS)
Diarrhea
Abdominal distension
Malabsorption
Affected mental and physical development
Reversible with Rx
Dx: Eggs in stool
Differential Diagnosis for Strongyloides
S. stercoralis → J1 in stool (No eggs!)
S. fulleborni → Eggs in stool
Trichuris trichiura
Distribution: Worldwide
DH: Humans (direct life cycle)
Transmission: Ingestion of eggs with J3
Egg with J3 → Small Intestine (J3) →→ Adult → Colon
Morphology:
Adults: Stichosome esophagus (thin head), wider body, whip-like appearance
Eggs: Bipolar plugs with a thick brown shell
Pathology: Feeds on mucosa and blood, invade colon epithelium
Usually in children
Acute dysentery
Prolapsed rectum with mucosal swelling
Chronic colitis
Dx: Eggs in feces (barrel-shaped eggs with polar plugs)
Trichinella spiralis
DH: Humans
Transmission: Ingestion of raw meat containing J1 in nurse cell complex (J1 = Newborn larvae)
Niche: Eyes, tongue, jaw, diaphragm, and intercostals
Reservoir: Pigs (zoonotic disease)
Urban cycle – pigs, rats, humans
Sylvan cycle – wild animals
Morphology: Worms are slightly more slender anterior than posterior
Female: larvae are visible in the uterus
Disease: Trichinosis or Trichinellosis
Enteral Phase (short duration) → Gastroenteritis, diarrhea
Parenteral Phase → Muscle pain, tenderness, eosinophilia
Dx: Muscle biopsy
Prevention: Cook your pork properly
T. spiralis Life Cycle
Enteral Phase: J1 → Columnar epithelium in upper SI →→ Adults [5 day post mating] → “newborn larvae“
Pathology: Short-term enteritis
Molts damage columnar epithelium
Adults thread through cells → Cell damage
Releasing NBL → Local inflammatory response
Parental Phase: J1 → Bloodstream → Enter cells → Skeletal muscle fibers
Nurse Cell Formation for J1
Pathology: Association between parasite and host resulting in nurse cell complex
Effect on the body:
Lose contractile myofilaments, DNA replication and becomes walled off
Alters gene expression; arrests DNA and redirect to benefit the parasite
Stimulates angiogenesis (blood vessel formation) called a “circulatory rete“ around nurse cell
Spirurina
Indirect life cycles
Vector: blood-sucking arthropod
Larvae are microfilaria, NOT J1
Microfilaria found in the bloodstream
Dx criteria: periodicity periods of high levels of microfilaria in circulation
Diurnal daytime (Loa loa)
Nocturnal night (W. bancrofti, Brugia sp)
Sexually dimorphic
Male small and posterior end has a corkscrew appearance
Female larger than male and is ovoviparous
J3 is infective stage for vertebrate host
Parasites of all vertebrates except fish
Wuchereria bancrofti
Distribution: Tropical and subtropical regions
Vector: Culicine and Anopheline mosquito
DH: Humans
Disease: Lymphatic Filariasis
Dx: Microfilariae in blood (night)
Brugia malayi
Distribution: India, Malaysia, and other parts of SE Asia
Vector: Mosquito (J1→J3)
DH: Humans (J3→ Sub Tissue → Lymphatic vessels → Adult → Microfilaria)
Niche: Lymphatic vessels
In the lumen of vessels especially in the upper and lower extremities
Genitalia of males
Morphology:
Adults
Thread-like
Females produce microfilaria
Produces 10,000 microfilaria per day
Microfilaria
Circulate within bloodstream
Infective stage for mosquito vector
Periodicity of microfilaremia: active at night
Pathology: Host inflammatory and immune response
Dx: Microfilaria in blood smears
Heavy infection – Thin stained smears
Light infection – Concentration of microfilaria (Knotts test) then stain the smear
OR detection of circulating adult antigens
Prevention: Vector control with insecticides
Clinical Symptoms of W. Bancrofti, Brugia sp
Endemic “normals”
No clinical signs
No microfilaremia detectable at any time
Asymptomatic microfilaremics
High leaves of microfilaria
No clinical signs
Acute lymphadenitis and filarial fevers
Circulating microfilaremia and recurrent bouts of fever and malaise with inflammation of the lymphatics including painful swollen lymph nodes
Chronic obstructive lymphadenitis
Repeated exposure to parasites
Elephantiasis – is the ultimate end point of chronic obstructive disease
Not everyone who develops chronic obstructive lymphadenitis → elephantiasis
Tropical pulmonary eosinophilia
Young men in southern India
Severe asthma-like syndrome with markedly elevated circulating eosinophilia
Onchocerca volvulus
Distribution: Africa, Central and South America (Mexico, Central American and regions of S. America)
Vector: Simulium sp “Black fly“
DH: Humans
Niche: Subcutaneous tissue of skin and eyes
Morphology:
Adults cluster and intertwine together in pairs or groups in nodules (onchocercomas) in the subcutaneous tissues of skin
Microfilaria has no sheath
Pathology: “River blindness“
Lesions in eyes and skin
Microfilaria or dying adults cause an inflammatory immune response
Dx: Biopsy of nodes or skin biopsy
NOT RECOMMENDED: Mazotti Test
Rx: Excision of nodules to decrease reaching the eye
Prevention: Vector control
Clinical Disease of Ochocerca volvulus
1) Onchodermatitis (skin dermatitis)
intense itching
Rash consisting of numerous elevated and very itchy papules
Skin becomes wrinkling, cracking and depigmentation: “leopard skin” or sowda (clinical term: lichenification)
2) Nodule formation around adults in skin:
Nodule formed is called “onchocercoma”
Antigenicity of worm products
Formation of fibrous nodules with a network of blood vessels [similar to rete formation of nurse cell induction in T. spiralis]
The host response is to encapsulate adults in this nodule
3) Ocular Lesions
All parts of the eye become affected in chronic, long-term infections
Conjunctivitis, keratitis, photophobia, and secondary glaucoma
Sclerosing keratitis (scarring and hardening of cornea) is a major cause of blindness
keratitis is a result of inflammatory response to microfilaria in eye; they can be found in choroid, retina, anterior chamber etc.
Another cause of blindness can be the immune response to microfilaria in retina
4) Lymphadenopathy
Loa Loa
Distribution: West Africa and Sudan
Vector: Crysops spp “deer fly“
DH: Human
Life Cycle: Similar to W. bancrofti
Pathology:
Asymptomatic
Calabar swelling and angioedema
Increased immune response
Elevated levels of IgE and eosinophilia
Antigen elicit response
Endemic normals
Develop protective immunity
Dx: Microfilaria in blood smears
Prevention: Treat to decrease transmission (repeating doses)
“Dog heartworm“
Vector: Mosquito
DH: Dogs
Life Cycle: Microfilaria → Mosquito → J1-J3 → Bloodstream → Heart and Pulmonary Arteries → Adults
Clinical Signs:
Soft cough and exercise intolerance
Cardiopulmonary failure → Death
Dx: Microfilaria in blood
Distinguish between Dirofilaria and Dipetalonema (nonpathogenic)
Prevention: Ivermectin (once a month)
Rx: Tricky, killing adults can cause fatal pulmonary emboli
Man can be an accidental host (not common)
Find “coin lesions“ in lungs; nodules with a dead worm
Dracunculus medinensis
“fiery serpent” or “guinea worm”
Distribution: Middle East and Africa (Only 5 countries still have the parasite)
Vector: Copepod (Cyclops sp)
DH: Humans
Life Cycle: J1 → Copepod → J3 → Ingestion by human → Small Intestine (J3) → Connective tissue →→ Adult
Female move to lower extremities to release J1 through an ulcer into water (no CLM)
Morphology: Females releases larvae in water
Pathology: Disfigures skin and subcutaneous tissues and site for 2nd bacterial infections
Ulcerated lesions → Tetanus, gangrene etc.
Dx: Lesion with worm
Rx: Extraction by winding worm on a stick until entire worm removed
Prevention: Boil water OR Filter with fine mesh
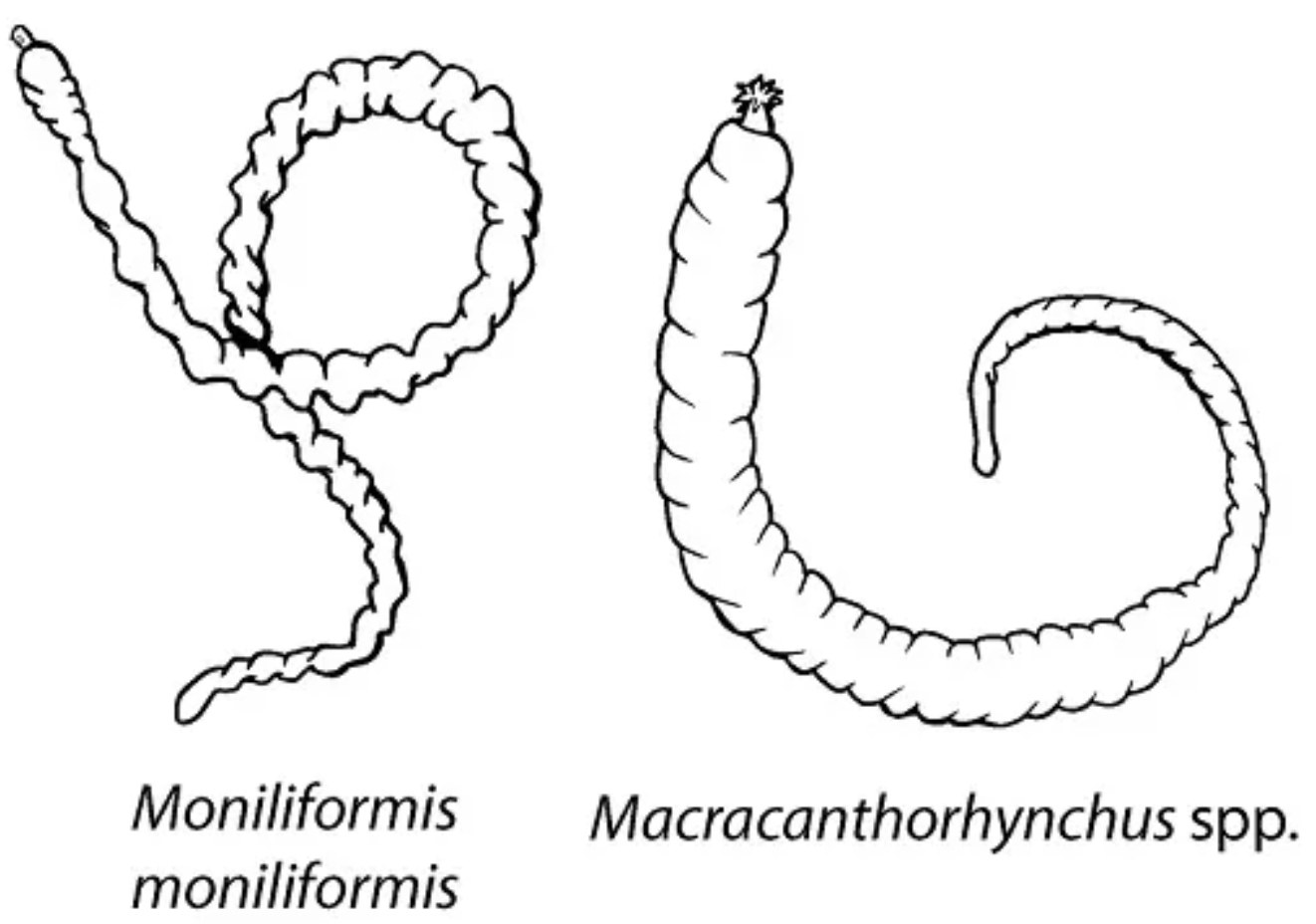
Macracanthorhynchus hirudinaceus
“Thorny-headed Worms“
IH: Beetles
Eats egg contain embryo called acanthor
Acanthor → Acanthella → Infective Stage: Cystacanth
DH: Pigs
Eats beetle with cystacanth → Gut wall with its proboscis into mucosa
Trauma and damage of attachment and movement to different locations
Penetration of gut wall → Fatal peritonitis
Morphology:
Anterior proboscis covered with tegument
Thin muscular wall with embedded recurved, sclerotized hooks
Fluid-filled
Extended and retracted into a muscular receptacle (sac)
Neck
Trunk
Moniliformis moniliformis
DH: Rats and other rodents