Properties of Water
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Covalent Bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule.
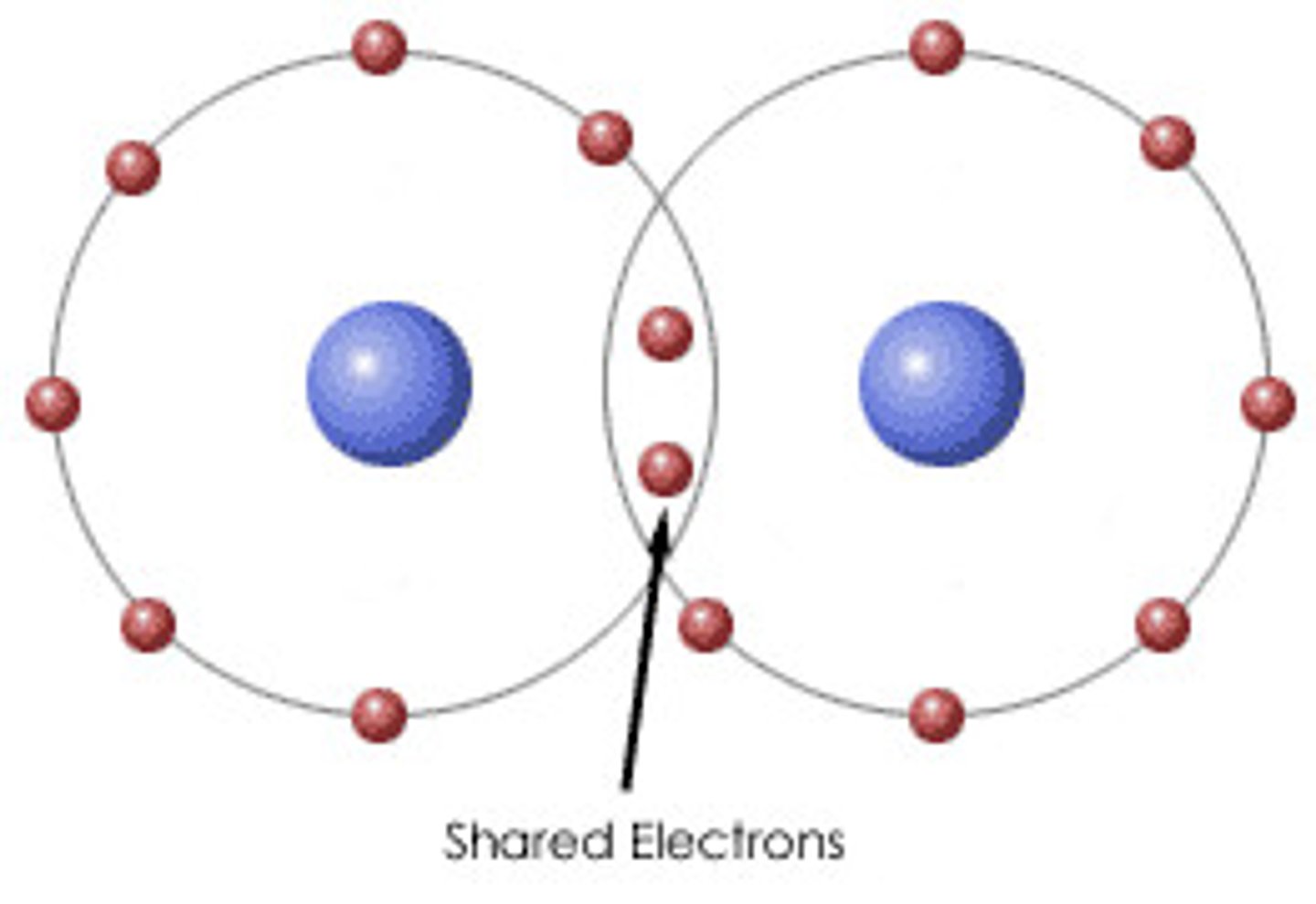
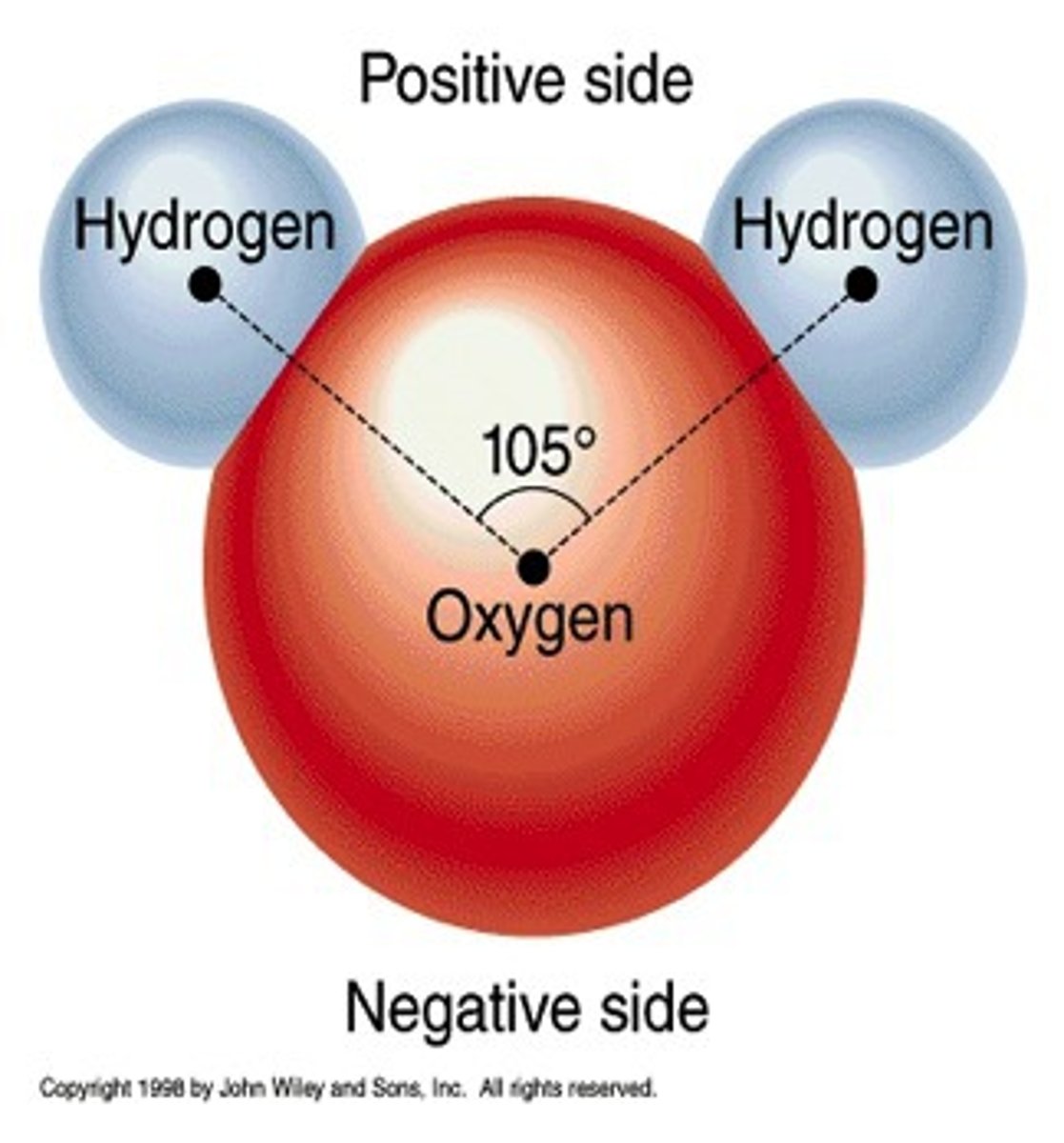
Polar Covalent Bond
A covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive.
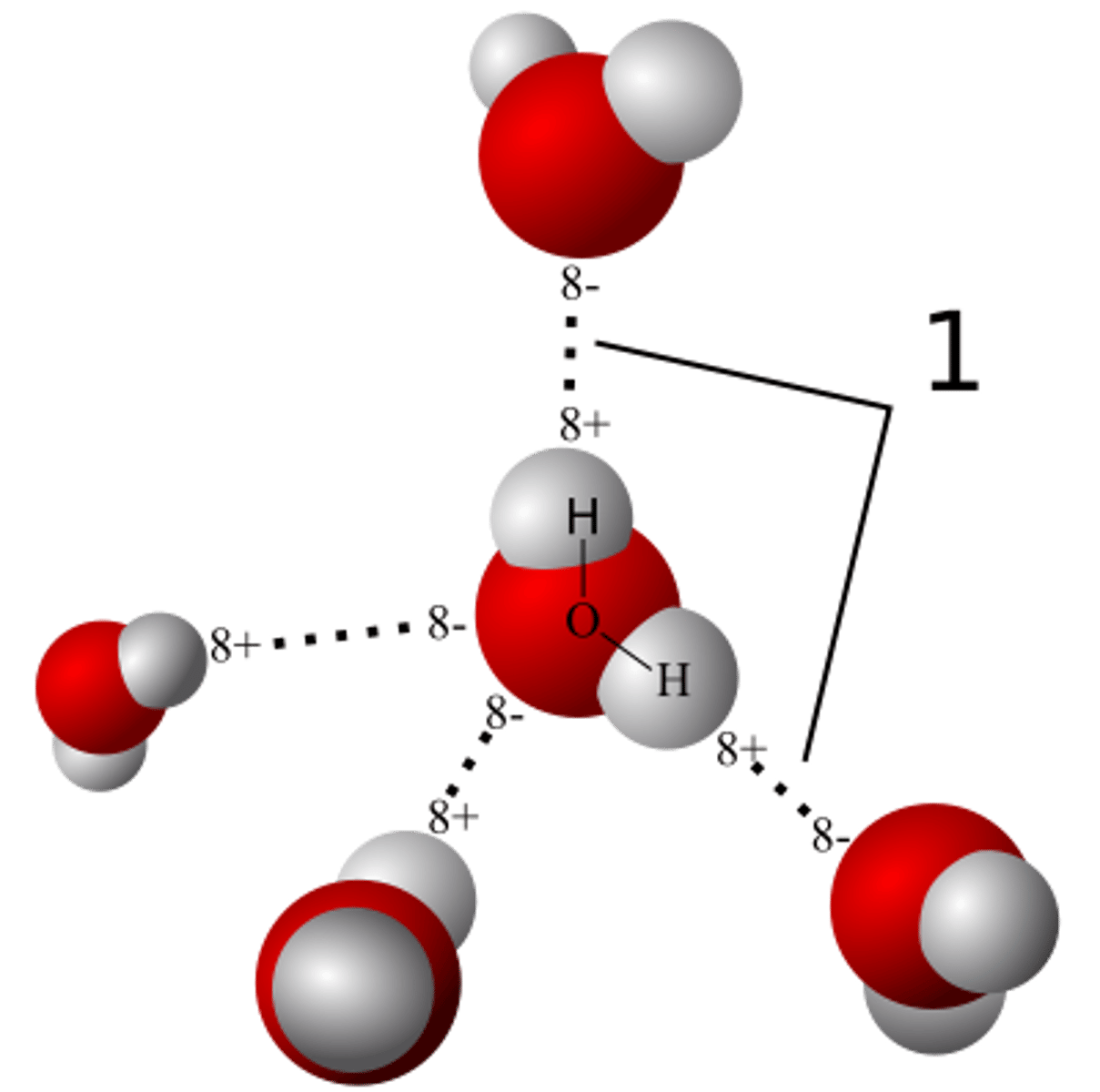
Hydrogen Bond
A type of weak chemical bond formed when the slightly positive hydrogen atom of a polar covalent bond in one molecule is attracted to the slightly negative atom of a polar covalent bond in another molecule.
Cohesion
Attraction between polar molecules of the same substance.

Adhesion
An attraction between polar molecules of different substances.

Surface Tension
The property of a liquid's surface to resist external forces due to the cohesive forces between its molecules.

Universal Solvent
Water- due to its polarity and ability to dissolve many different solutes

Specific Heat Capacity
the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius

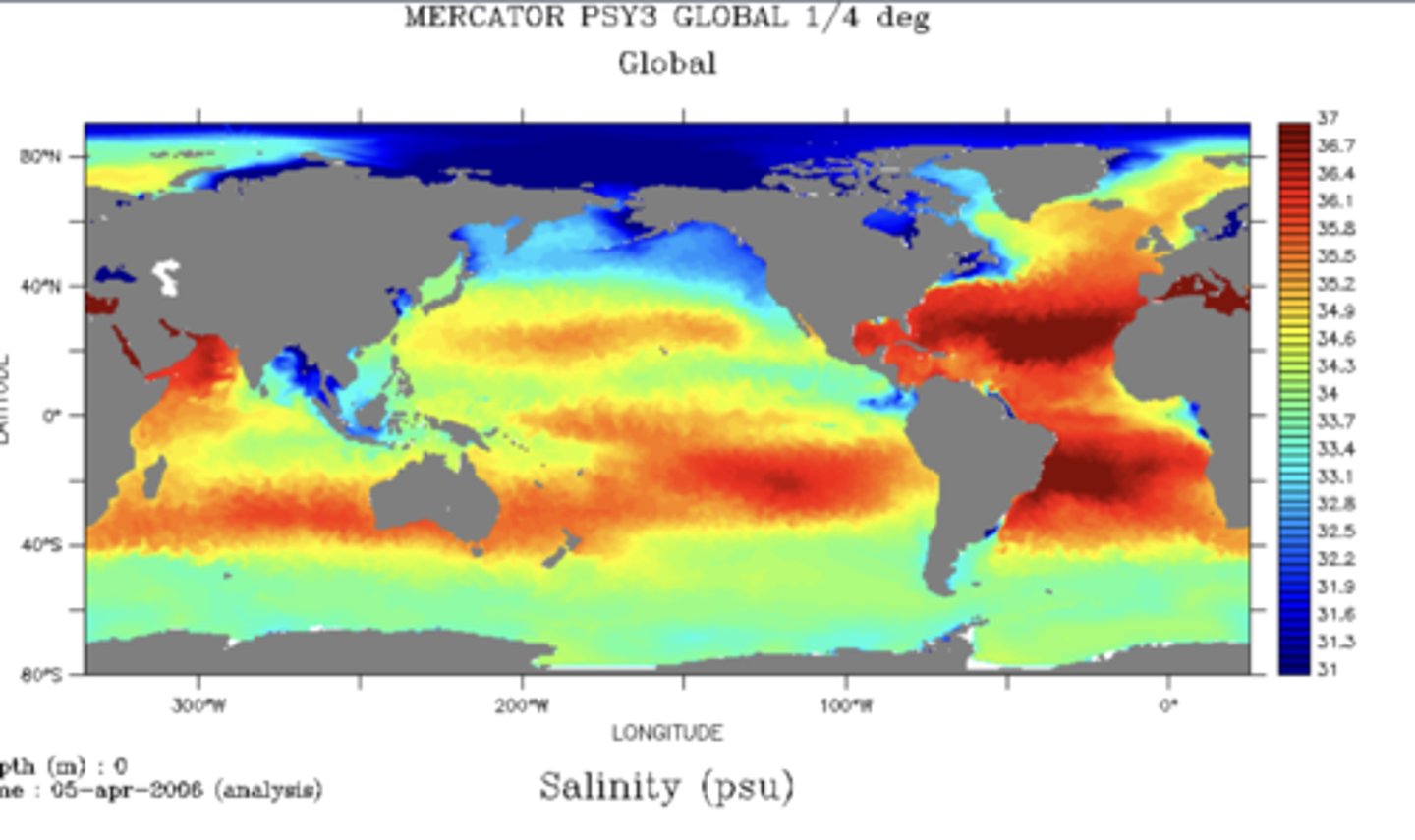
Salinity
The total amount of dissolved salts in a water sample. Measured in parts per thousand.

Sea Surface Salinity
Varies mostly by losses or gains of fresh water by evaporation or precipitation.